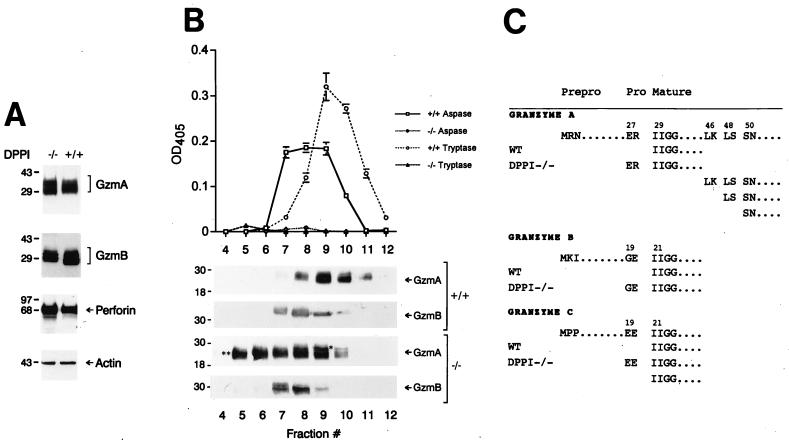
Figure 3.
Lack of granzyme processing and activity in LAK cells derived from DPPI−/− mice. (A) Granzyme A and B levels are normal in DPPI−/− LAK cells. Equal amounts of protein derived from wild type DPPI−/− LAK cells were analyzed by Western blotting by using polyclonal antibodies directed against mouse granzymes A and B and mAbs against mouse perforin and β-actin. The specificities of these antibodies have been determined previously (see Materials And Methods). (B) Granzyme A and B activities in LAK cell lysates. Wild-type and DPPI−/− LAK cell lysates were fractionated on a cationic exchange column by using a linear 0.25–2 M NaCl gradient, and each fraction was analyzed for aspase and tryptase activity. These same fractions were separated by SDS/PAGE, transferred to nitrocellulose, and incubated with anti-granzyme A and B antibodies to confirm the abundance and sizes of the proteins. (C) Granzymes are abnormally processed in DPPI−/− LAK cells. Column fractions from B were fractionated by using nonreducing SDS/10% PAGE and transferred to poly(vinylidene difluoride) membranes; specific bands were excised, eluted, and subjected to microsequencing to determine the N termini. These results were confirmed in two independent experiments.