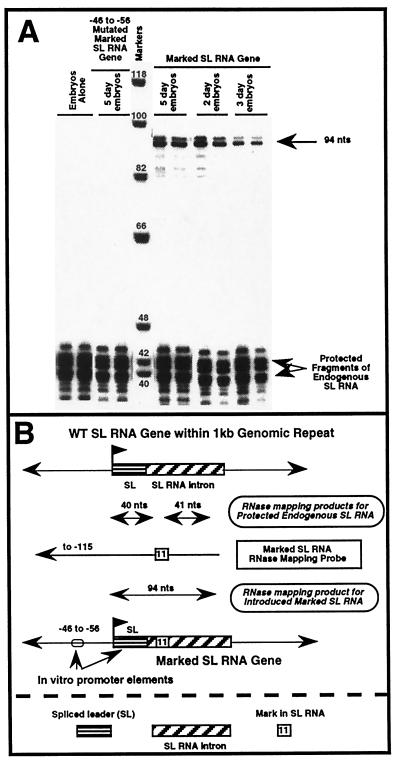
Figure 1.
Expression of a marked SL RNA gene in Ascaris embryos. (A) RNase mapping. Sixteen hours after bombardment of Ascaris embryos (2-day = ≈two cells, 3-day = ≈4–8 cells, or 5-day = ≈32–64 cells) with a marked SL RNA gene, total RNA was isolated and subjected to RNase mapping. The expected RNase mapping product for accurate initiation and expression of the marked SL RNA is 94 nt and is indicated by the upper, single arrow. Mapping of the endogenous wild-type SL RNA generates two fragments because of the mark (11-nt substitution) in the mapping probe (see B). Note that no endogenous RNAs of 94 nt are protected in untransfected or mock-transfected embryos (Embryos Alone). Embryos transfected with an SL RNA gene mutated with a block substitution from −46 to −56 of the SL RNA promoter show dramatically reduced levels of the marked RNA, similar to observations made in 32- to 64-whole-cell Ascaris embryo extracts (14). Data shown are representative of multiple experiments. (B) RNase mapping strategy and probe.