Figure 1.
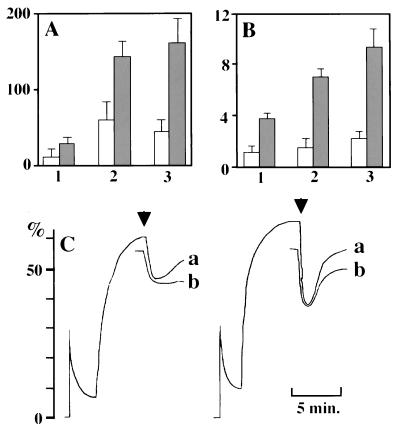
Functional defects in neutrophils from the patient. (A) Chemotactic ability assessed by Boyden chamber method toward 10−8 (1) or 10−9 M fMLP (2) or 10% zymosan-activated serum (3). Vertical numbers indicate numbers of cells in a field. We used different concentrations of fMLP because we found previously that concentrations of fMLP necessary for maximum chemotactic ability were different between the cells from cord and adult blood. Chemotactic ability of cord blood neutrophils was 70–80% of normal adults at an optimum concentration of fMLP for adult cells. In each concentration, the cells from the patient exhibited significant chemotaxis deficiency even compared with the ability of the cells from cord blood. (B) Maximum rate of superoxide generation assayed on exposure to 2 × 10−7 M fMLP (1), 2 × 10−7 M fMLP plus 2.5 μg/ml cytochalasin D (2), or 2.5 μg/ml cytochalasin D plus 50 μg/ml Con A (3). Vertical numbers indicate nanomoles of superoxide/minute/106 neutrophils. Open column, patient; closed column, control. (C) Relative change of fluorescence as an indication of depolarization and repolarization of patient (Left) and control neutrophils (Right). Di-O-C5 [3] was used as the lipophilic probe, and 2 × 10−7 M fMLP was used as a stimulus (arrows). Neutrophils immediately after isolation (a) and those stored on ice for 1 hour (b) were used.