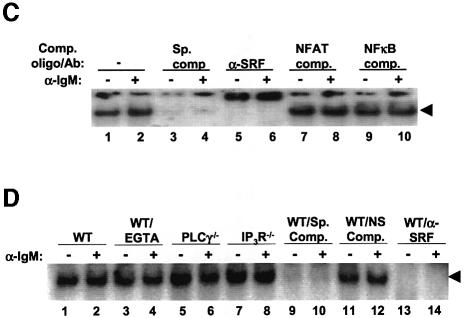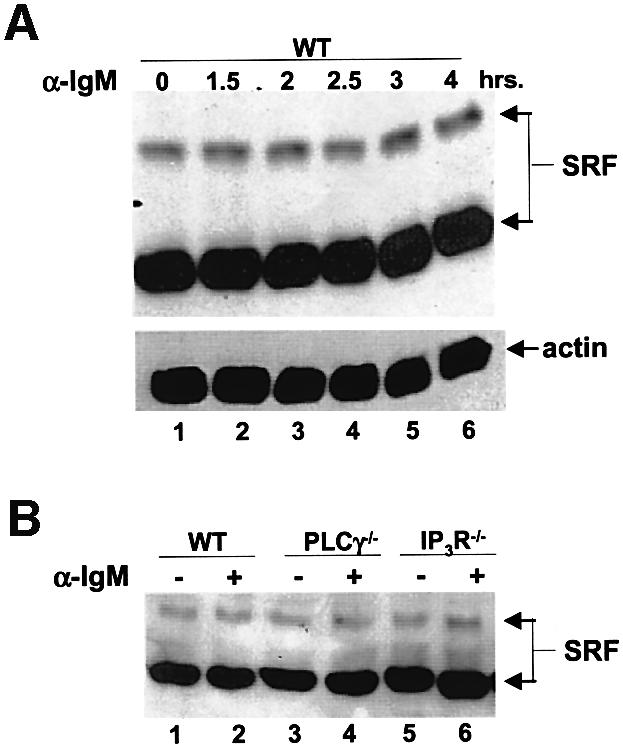
Fig. 8. SRF expression and DNA binding activity does not change during BCR signaling. (A) WT DT40 cells were either not stimulated (lane 1) or stimulated for the indicated times with anti-IgM, and lysates probed for SRF (top panel) or actin as a loading control (bottom panel) by western blotting. Note that we detect two forms of SRF in these chicken cells similar to previously published reports (Croissant et al., 1996). (B) SRF expression is not affected by the lack of PLCγ or IP3R. WT (lanes 1 and 2), PLCγ–/– (lanes 3 and 4) or IP3R–/– (lanes 5 and 6) were either left unstimulated (lanes 1, 3 and 5) or stimulated for 4 h with anti-IgM (lanes 2, 4 and 6), and lysates probed for SRF. Probing for actin as a loading control showed that there was equal amount of proteins in each lane (data not shown). (C) SRF DNA binding is not altered by BCR stimulation. WT DT40 cells were either not stimulated (odd numbered lanes) or stimulated for 1 h with anti-IgM (even numbered lanes), nuclear extracts prepared and EMSA performed. Extracts in lanes 3 and 4 were incubated in the presence of 50-fold excess of cold SRF oligo. Extracts in lanes 5 and 6 were incubated with antibodies to SRF. Extracts in lanes 7–10 were incubated with unlabeled oligos containing binding sites for NFAT (lanes 7 and 8) or NF-κB (lanes 9 and 10) as non-specific competitors. (D) The presence of EGTA or absence of PLCγ or IP3R does not affect DNA binding ability of SRF. WT (lanes 1–4, 9–14), PLCγ–/– (lanes 5 and 6) or IP3R–/– DT40 cells (lanes 7 and 8) were either not stimulated (odd numbered lanes) or stimulated for 10 min with anti-IgM (even numbered lanes), nuclear extracts prepared and EMSA performed. Note that in lanes 3 and 4, WT DT40 cells were incubated in the presence of 1 mM EGTA. Extracts in lanes 9 and 10 were incubated in the presence of 50-fold excess of cold SRF binding site oligo. Extracts in lanes 11 and 12 were incubated with 50-fold excess of cold NFAT binding site oligo. Extracts in lanes 13 and 14 were incubated with antibodies to SRF. The arrow indicates the SRF-specific DNA complex.