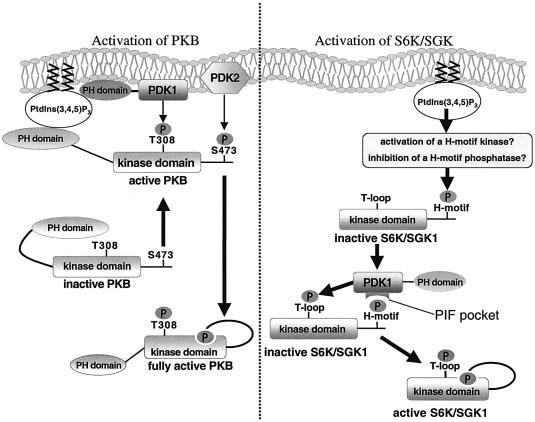
Fig. 8. Summary of the mechanism of activation of PKB, S6K and SGK by PDK1. PKB is activated following its recruitment to the plasma membrane where it is phosphorylated at Thr308 by PDK1 and at Ser473 by a distinct unknown hydrophobic motif kinase, termed PDK2. The mutual binding of PKB and PDK1 through their PH domains co-localizes PDK1 and PKB. Once PKB is phosphorylated at Thr308, a hydrophobic motif-binding site is formed in the catalytic domain, resulting in the intramolecular binding of the PKB hydrophobic motif phosphorylated at Ser473 to this site. This is the step that leads to the maximal activation of PKB. In contrast, for S6K and SGK, it is the phosphorylation of these enzymes at their hydrophobic motif that enables PDK1 to interact through its PIF-pocket and hence phosphorylate the T-loop of these substrates. PI-3-kinase regulates the phosphorylation of S6K and SGK1 at their hydrophobic motif. Phosphorylation of the T-loop of S6K and SGK is predicted to promote the formation of a binding site within the kinase domain of these enzymes, for their own phosphorylated hydrophobic motif, leading to activation. We propose that this protects dephosphorylation of the hydrophobic motif by protein phosphatases. In PDK1155E/155E cells, S6K is not phosphorylated at its T-loop and therefore the hydrophobic motif phosphorylation site will remain exposed and is thus more likely to be dephosphorylated.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.