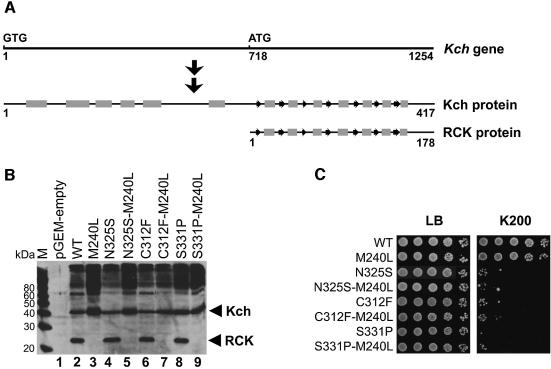
Fig. 6. Removing the internal start codon eliminates the RCK protein but does not suppress the toxicity of the GOF mutations. (A) Diagram of the relative position of the internal start and the two translation products, Kch and RCK protein. The protein secondary structures are indicated as gray rectangles (α-helix) and arrows (β-sheet). (B) Western blot analysis of the Kch proteins. The samples were prepared by directly boiling the stationary cells in SDS–PAGE sample buffer. As expected, both the larger (Kch) and the smaller (RCK) proteins are found in the wild-type (lane 2) and the GOF mutants (lanes 4, 6 and 8). Genetically engineered removal of the internal translational initiation removes the smaller peptide in both the wild-type (lane 3) and the GOF mutants (lanes 5, 7 and 9). M: MagicMark (Invitrogen, CA). (C) Growth analysis. Removal of the RCK proteins does not alter the growth phenotype of the wild-type (rows 1 and 2). It also does not affect the toxic phenotype of the GOF mutants (rows 3–8).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.