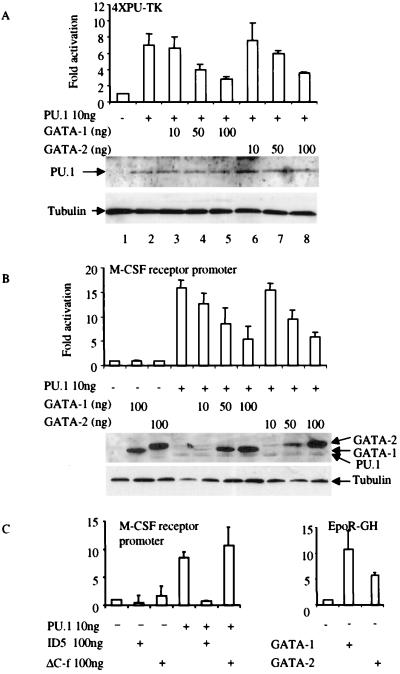
Figure 3.
GATA-1 and GATA-2 repress PU.1 transactivation. (a) CV-1 cells were transfected with 100 ng of the multimerized PU.1-binding-site reporter (4XPU-TK), 10 ng of PU.1 expression vector PU.PECE (31), and GATA-1 or GATA-2 expression vectors as indicated. Luciferase activity is shown as fold activation above the level of activity seen with the luciferase reporter in the absence of any added PU.1 expression vector (±SD; n = 4). Western blot analysis was performed by using anti-PU.1 antibody to detect the expression level of PU.1 protein in transfected CV-1 cells as shown, with antitubulin antibody as a loading control. (b) GATA-1 and GATA-2 inhibit PU.1 transactivation of the M-CSF receptor promoter. CV-1 cells were transfected with 100 ng of M-CSF receptor promoter-luciferase, 10 ng of PU.PECE, and GATA-1 or GATA-2 expression vectors as indicated. Luciferase activity is shown as fold activation (±SD; n = 4). GATA-1 or GATA-2 alone did not repress the basal activity of either the 4XPU-TK (a) or the M-CSF receptor promoter (b). Western blot analysis was performed by using anti-FLAG antibody to detect the expression levels of FLAG-tagged PU.1, GATA-1, and GATA-2 proteins in transfected CV-1 cells as shown, with antitubulin antibody as a loading control. (c) Deletion mutation of the C finger of GATA-1 loses the ability to inhibit PU.1 function. (Left) CV-1 cells were transfected with the M-CSF receptor promoter and PU.1, ID5, and ΔC-f expression vectors as indicated. Luciferase activity is shown as fold activation (±SD; n = 3). (Right) The reporter is the erythropoietin receptor promoter-driving human growth hormone (EpoR-GH). Four micrograms of EpoR-GH and 9 μg of GATA-1 and GATA-2 were used in these transfection experiments. ID5, N-terminal deletion (amino acids 1–63 were deleted) of GATA-1; ΔC-f, C finger deletion mutation of GATA-1.