Abstract
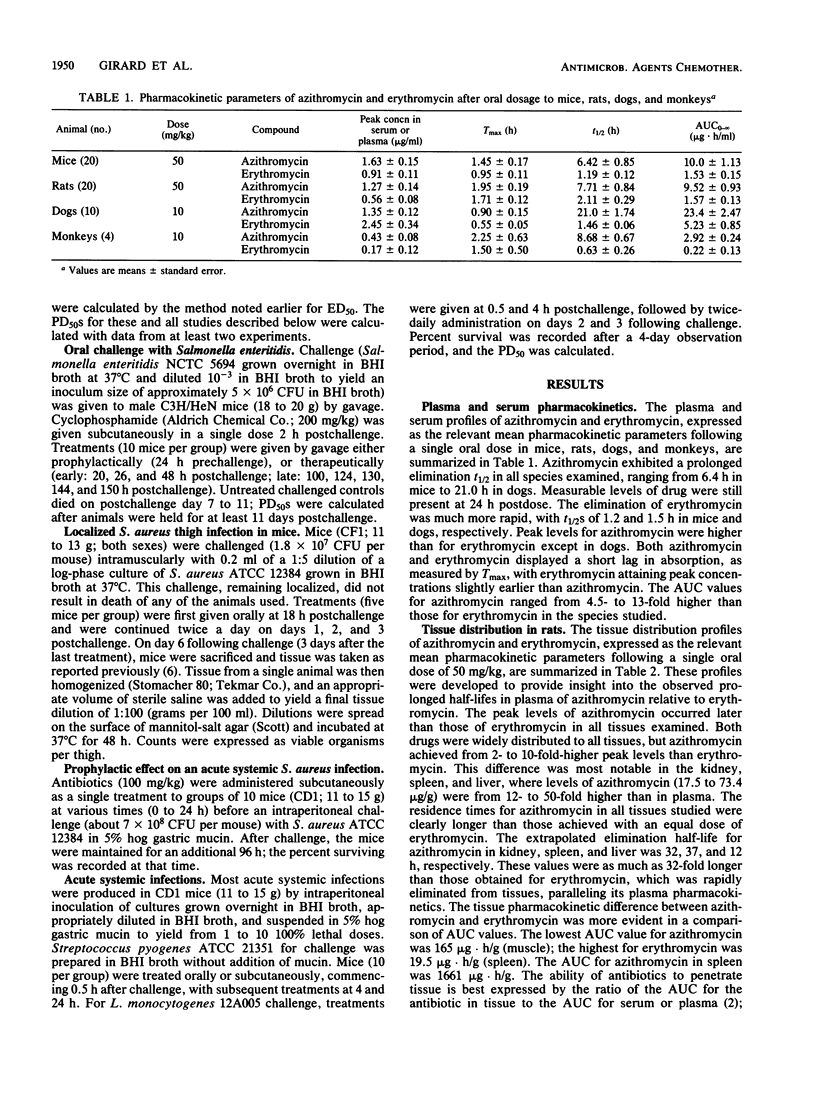
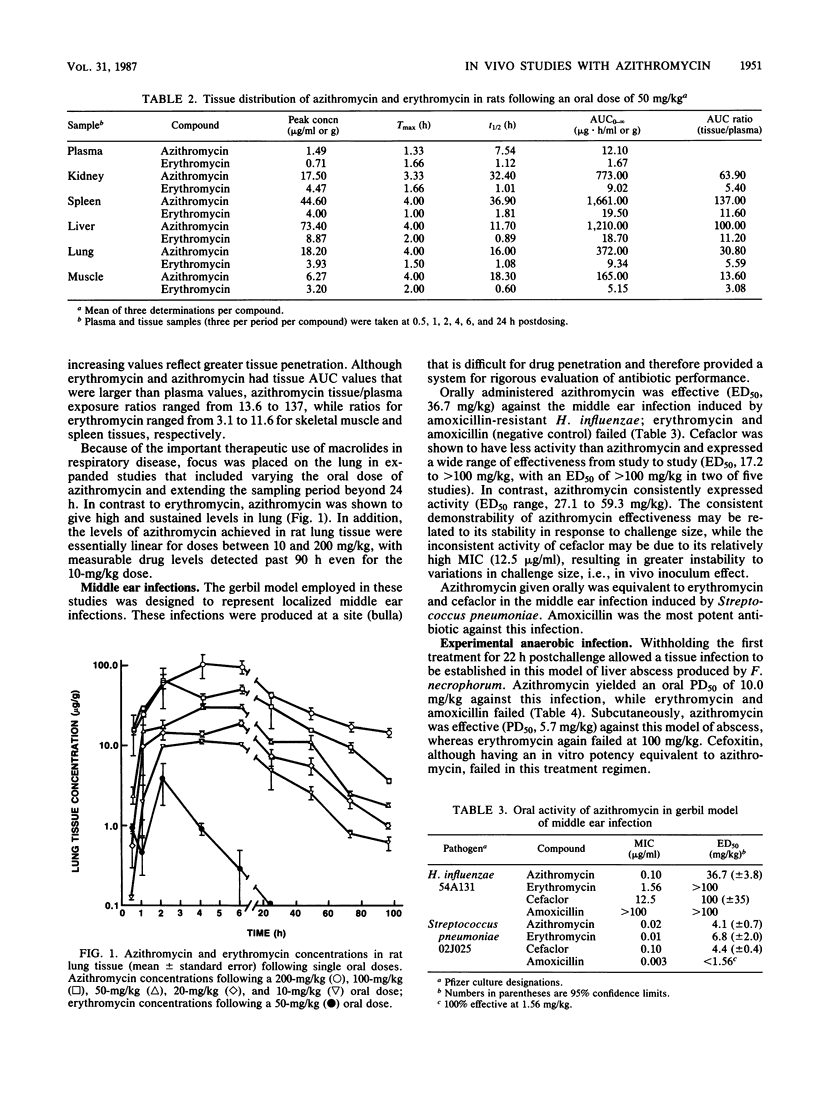
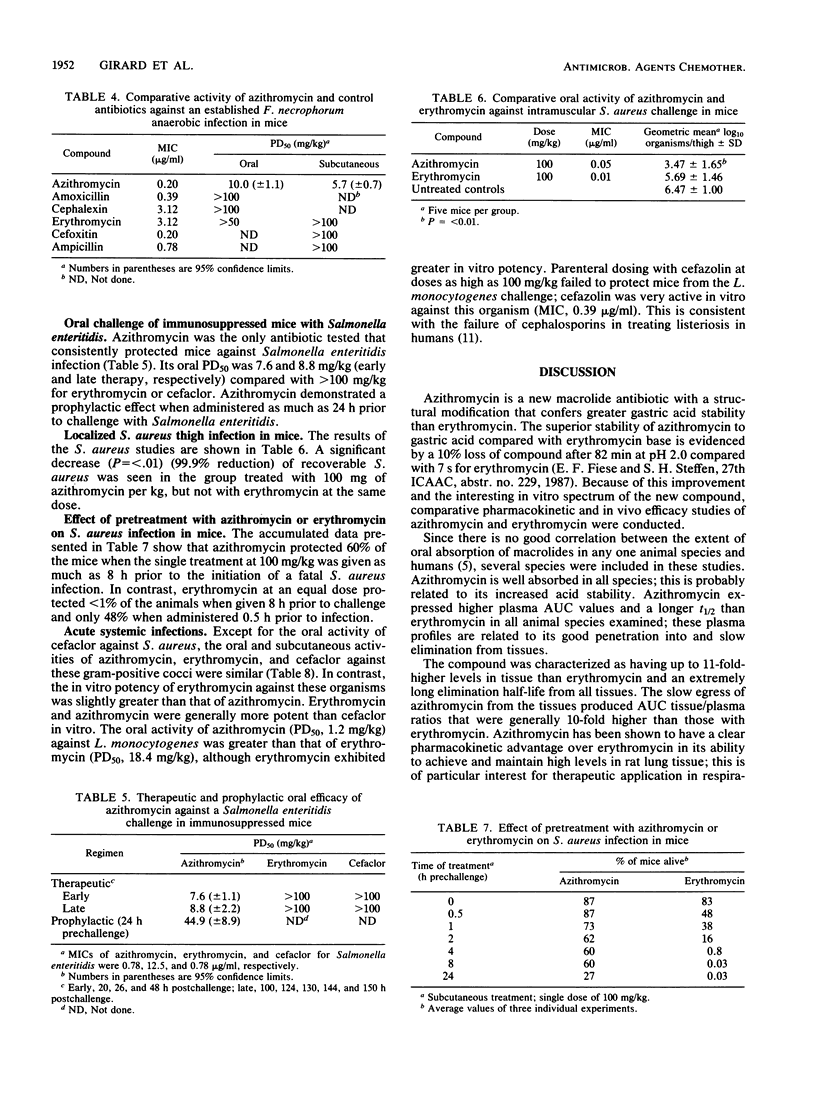
Azithromycin (CP-62,993), a new acid-stable 15-membered-ring macrolide, was well absorbed following oral administration in mice, rats, dogs, and cynomolgus monkeys. This compound exhibited a uniformly long elimination half-life and was distributed exceptionally well into all tissues. This extravascular penetration of azithromycin was demonstrated by tissue/plasma area-under-the-curve ratios ranging from 13.6 to 137 compared with ratios for erythromycin of 3.1 to 11.6. The significance of these pharmacokinetic advantages of azithromycin over erythromycin was shown through efficacy in a series of animal infection models. Azithromycin was orally effective in treating middle ear infections induced in gerbils by transbulla challenges with amoxicillin-resistant Haemophilus influenzae or susceptible Streptococcus pneumoniae; erythromycin failed and cefaclor was only marginally active against the H. influenzae challenge. Azithromycin was equivalent to cefaclor and erythromycin against Streptococcus pneumoniae. In mouse models, the new macrolide was 10-fold more potent than erythromycin and four other antibiotics against an anaerobic infection produced by Fusobacterium necrophorum. Similarly, azithromycin was effective against established tissue infections induced by Salmonella enteritidis (liver and spleen) and Staphylococcus aureus (thigh muscle); erythromycin failed against both infections. The oral and subcutaneous activities of azithromycin, erythromycin, and cefaclor were similar against acute systemic infections produced by Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus viridans, or S. aureus, whereas azithromycin was more potent than erythromycin and cefaclor against the intracellular pathogen Listeria monocytogenes. The pharmacokinetic advantage of azithromycin over erythromycin in half-life was clearly demonstrated in prophylactic treatment of an acute mouse model of S. aureus infection. These properties of azithromycin strongly support the further evaluation of this new macrolide for use in community-acquired infections of skin or soft tissue and respiratory diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergan T. Pharmacokinetics of tissue penetration of antibiotics. Rev Infect Dis. 1981 Jan-Feb;3(1):45–66. doi: 10.1093/clinids/3.1.45. [DOI] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H. J., 3rd, Fulghum R. S., Brinn J. E., Barrett K. A. Comparative anatomy of eustachian tube and middle ear cavity in animal models for otitis media. Ann Otol Rhinol Laryngol. 1982 Jan-Feb;91(1 Pt 1):82–89. doi: 10.1177/000348948209100118. [DOI] [PubMed] [Google Scholar]
- Duthu G. S. Interspecies correlation of the pharmacokinetics of erythromycin, oleandomycin, and tylosin. J Pharm Sci. 1985 Sep;74(9):943–946. doi: 10.1002/jps.2600740907. [DOI] [PubMed] [Google Scholar]
- English A. R., Girard D., Cimochowski C., Faiella J., Retsema J. A., Lynch J. E. Activity of sulbactam/ampicillin in screening and discriminative animal models of infection. Rev Infect Dis. 1986 Nov-Dec;8 (Suppl 5):S535–S542. doi: 10.1093/clinids/8.supplement_5.s535. [DOI] [PubMed] [Google Scholar]
- English A. R., Girard D., Haskell S. L. Pharmacokinetics of sultamicillin in mice, rats, and dogs. Antimicrob Agents Chemother. 1984 May;25(5):599–602. doi: 10.1128/aac.25.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A. R., Girard D., Retsema J. A. Pirbenicillin: pharmacokinetic parameters in mice. Antimicrob Agents Chemother. 1976 Sep;10(3):491–497. doi: 10.1128/aac.10.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulghum R. S., Brinn J. E., Smith A. M., Daniel H. J., 3rd, Loesche P. J. Experimental otitis media in gerbils and chinchillas with Streptococcus pneumoniae, Haemophilus influenzae, and other aerobic and anaerobic bacteria. Infect Immun. 1982 May;36(2):802–810. doi: 10.1128/iai.36.2.802-810.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L., Steinberg T. H. Interactions of antibiotics and phagocytes. J Antimicrob Chemother. 1983 Oct;12 (Suppl 100):1–11. doi: 10.1093/jac/12.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- Kawaler B., Hof H. Failure of cephalosporins to cure experimental listeriosis. J Infect. 1984 Nov;9(3):239–243. doi: 10.1016/s0163-4453(84)90486-9. [DOI] [PubMed] [Google Scholar]
- Klein J. O. Microbiology of otitis media. Ann Otol Rhinol Laryngol Suppl. 1980 May-Jun;89(3 Pt 2):98–101. doi: 10.1177/00034894800890s326. [DOI] [PubMed] [Google Scholar]
- Prokesch R. C., Hand W. L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982 Mar;21(3):373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. J., Yin E. J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970 Apr;19(4):573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Wilson W. R. Erythromycin: a microbial and clinical perspective after 30 years of clinical use (1). Mayo Clin Proc. 1985 Mar;60(3):189–203. doi: 10.1016/s0025-6196(12)60219-5. [DOI] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Wilson W. R. Erythromycin: a microbial and clinical perspective after 30 years of clinical use (2). Mayo Clin Proc. 1985 Apr;60(4):271–278. doi: 10.1016/s0025-6196(12)60322-x. [DOI] [PubMed] [Google Scholar]
- Wilkins T. D., Smith L. D. Chemotherapy of an experimental Fusobacterium (Sphaerophorus) necrophorum infection in mice. Antimicrob Agents Chemother. 1974 Jun;5(6):658–662. doi: 10.1128/aac.5.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. T., van Boxtel C. J. Pharmacokinetics of Erythromycin in man. Antibiot Chemother (1971) 1978;25:181–203. [PubMed] [Google Scholar]


