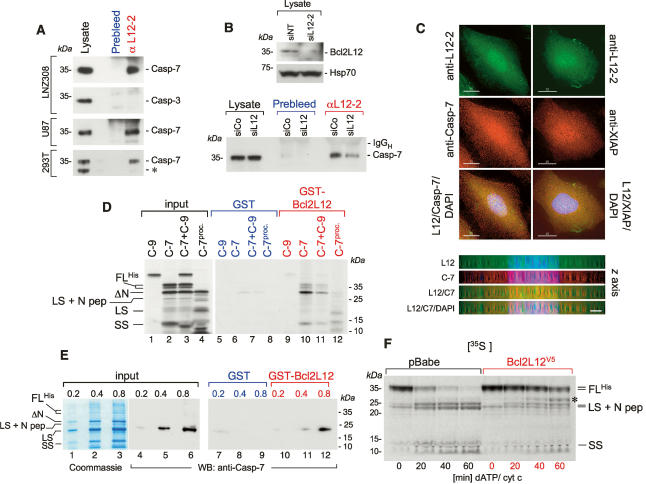
Figure 5.
Bcl2L12 directly interacts with caspase-7 in vitro and in vivo. (A) Anti-L12-2 immunoprecipitates from lysates of LNZ308, U87MG, and 293T cells were subjected to Western blot analysis for procaspase-7 and procaspase-3. For the lysate lane, one-fortieth of the lysate used for the IP was loaded. The asterisk-marked band in the lysates of 293T cells most likely represents an N-terminally truncated caspase-7 enzyme. (B, top) Anti-L12-2 immunoprecipitates from siNT- and siL12-2-transfected U87MG cells with documented knockdown of Bcl2L12 by Western blot were subjected to Western blot analysis for caspase-7. As in A, one-fortieth of the lysate was loaded in the lysate lanes. Hsp70 is shown as a loading control for the Bcl2L12 Western blot. (C) LNZ308 cells were subjected to deconvolution microscopy using the anti-L12-2 antiserum (top row), a monoclonal anti-caspase-7 antibody (left panel, center row), and a monoclonal anti-XIAP antibody (right panel, center row). (Bottom row) Green and red images were overlaid together with DAPI stainings. Bar, 15 μm. (Bottom panel) Deconvoluted images for the Bcl2L12/caspase-7 staining were rotated by 90° along the X-axis to analyze Bcl2L12 and caspase-7 distribution along the Z-axis. Bar, 3 μm. (D) In vitro translated caspase-9 (C-9), caspase-7 (C-7) with a C-terminal His-tag, a mixture of caspase-9 and caspase-7 (C-9 + C-7), and processed caspase-7 (in vitro translated caspase-7 preincubated with 2.8 nM recombinant active caspase-3) were incubated with GST or GST-Bcl2L12 coupled to GSH beads. Precipitates were subjected to SDS-PAGE followed by autoradiography. The migration positions of caspase-7 and of the active caspase-7 subunits are indicated. (FLHis) (His)6-tagged full-length caspase-7; (LS) large subunit; (SS) small subunit; (N pep) N-terminal peptide spanning amino acids 1–23. (E) GST or GST-Bcl2L12 was incubated with increasing amounts of soluble recombinant (His)6-tagged caspase-7, and subjected to SDS-PAGE followed by Coomassie staining or by Western blot analysis using an anti-caspase-7 antibody. (Lanes 1–3) Due to immediate autoproteolysis of procaspase-7 upon protein induction in bacteria (Stennicke and Salvesen 1999), the proenzyme was nearly completely converted into cleavage intermediates. The migration positions of the caspase-7 species are indicated. (F) Lysates from pBabe- and Bcl2L12-expressing astrocyte cultures were activated with dATP (1 mM) and cytochrome c (5 μM), and the processing of in vitro translated caspase-7 was followed by autoradiography. Of note, an in vitro translated band at ∼32 kDa accumulated in stimulated Bcl2L12 lysates (marked with an asterisk) that could possibly represent ΔN-caspase-7, which has been shown to only have minor catalytic (DEVDase) activity (Denault et al. 2006).