Abstract
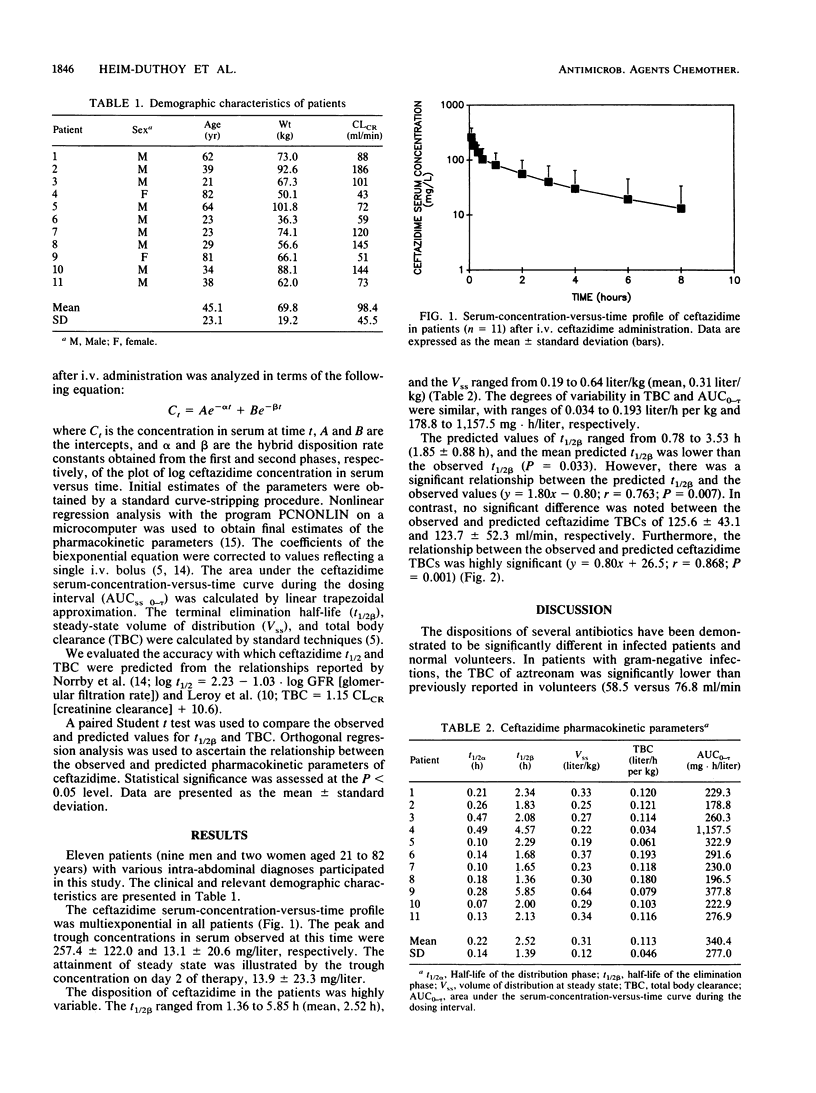
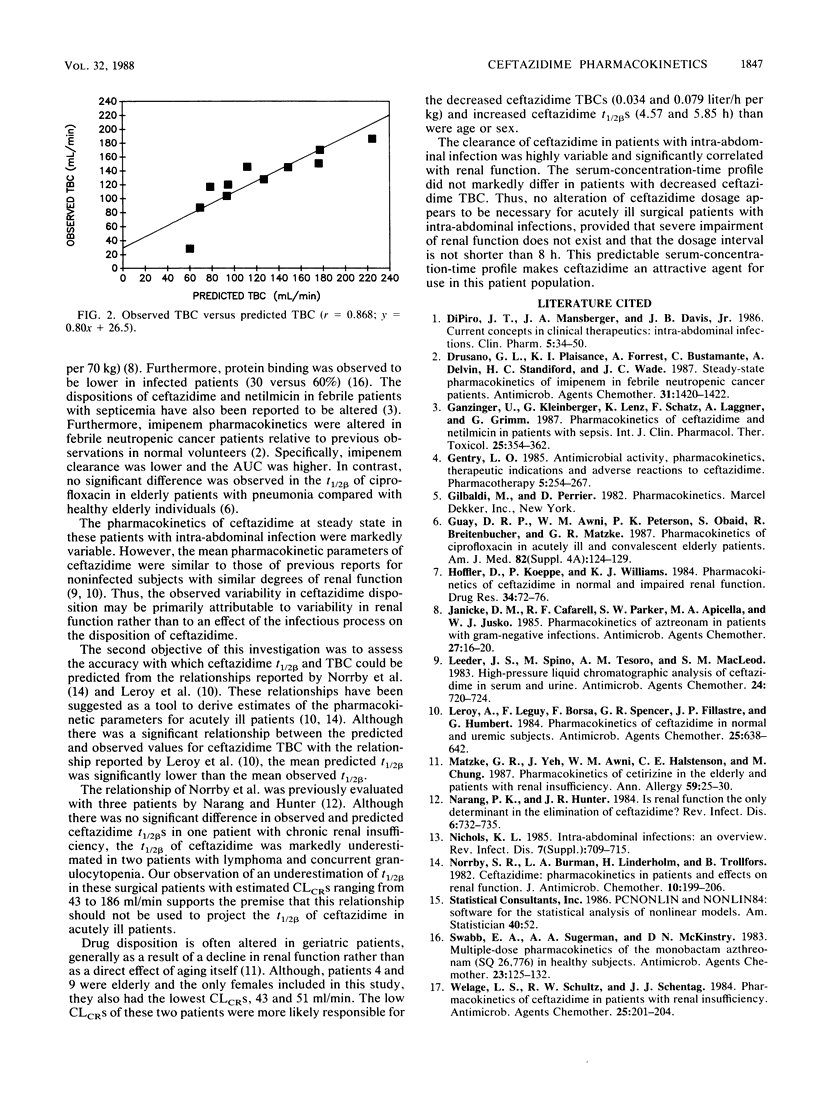
The disposition of ceftazidime was assessed in 11 surgical patients with suspected intra-abdominal infection. All patients had normal hepatic function, and creatinine clearances ranged from 43 to 186 ml/min. Patients received 2 g of ceftazidime intravenously every 8 h. Trough and peak concentrations in serum were measured on day 2, and trough and postdose concentrations in serum were determined on 10 samples collected during a dosage interval between days 3 and 6 of therapy. Ceftazidime peak and trough concentrations in serum at steady state determined by high-performance liquid chromatography were 257.4 +/- 122.0 (mean +/- standard deviation) and 13.1 +/- 20.6 mg/liter. The serum-concentration-versus-time profile was multiexponential. The elimination half-life, steady-state volume of distribution, and total body clearance were 2.52 +/- 1.39 h, 0.31 +/- 0.12 liter/kg, and 0.11 +/- 0.05 liter/h per kg, respectively. Total predicted body clearance significantly correlated with the measured values (r = 0.868; P = 0.001). The disposition of ceftazidime is dependent on creatinine clearance and is not significantly altered by surgery or acute infectious processes.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DiPiro J. T., Mansberger J. A., Davis J. B., Jr Current concepts in clinical therapeutics: intra-abdominal infections. Clin Pharm. 1986 Jan;5(1):34–50. [PubMed] [Google Scholar]
- Drusano G. L., Plaisance K. I., Forrest A., Bustamante C., Devlin A., Standiford H. C., Wade J. C. Steady-state pharmacokinetics of imipenem in febrile neutropenic cancer patients. Antimicrob Agents Chemother. 1987 Sep;31(9):1420–1422. doi: 10.1128/aac.31.9.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzinger U., Kleinberger G., Lenz K., Schatz F., Laggner A., Grimm G. Pharmacokinetics of ceftazidime and netilmicin in patients with sepsis. Int J Clin Pharmacol Ther Toxicol. 1987 Jul;25(7):354–362. [PubMed] [Google Scholar]
- Gentry L. O. Antimicrobial activity, pharmacokinetics, therapeutic indications and adverse reactions of ceftazidime. Pharmacotherapy. 1985 Sep-Oct;5(5):254–267. doi: 10.1002/j.1875-9114.1985.tb03424.x. [DOI] [PubMed] [Google Scholar]
- Guay D. R., Awni W. M., Peterson P. K., Obaid S., Breitenbucher R., Matzke G. R. Pharmacokinetics of ciprofloxacin in acutely ill and convalescent elderly patients. Am J Med. 1987 Apr 27;82(4A):124–129. [PubMed] [Google Scholar]
- Höffler D., Koeppe P., Williams K. J. Pharmacokinetics of ceftazidime in normal and impaired renal function. Arzneimittelforschung. 1984;34(1):72–76. [PubMed] [Google Scholar]
- Janicke D. M., Cafarell R. F., Parker S. W., Apicella M. A., Jusko W. J. Pharmacokinetics of aztreonam in patients with gram-negative infections. Antimicrob Agents Chemother. 1985 Jan;27(1):16–20. doi: 10.1128/aac.27.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeder J. S., Spino M., Tesoro A. M., MacLeod S. M. High-pressure liquid chromatographic analysis of ceftazidime in serum and urine. Antimicrob Agents Chemother. 1983 Nov;24(5):720–724. doi: 10.1128/aac.24.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A., Leguy F., Borsa F., Spencer G. R., Fillastre J. P., Humbert G. Pharmacokinetics of ceftazidime in normal and uremic subjects. Antimicrob Agents Chemother. 1984 May;25(5):638–642. doi: 10.1128/aac.25.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke G. R., Yeh J., Awni W. M., Halstenson C. E., Chung M. Pharmacokinetics of cetirizine in the elderly and patients with renal insufficiency. Ann Allergy. 1987 Dec;59(6 Pt 2):25–30. [PubMed] [Google Scholar]
- Narang P. K., Hunter J. R. Is renal function the only determinant in the elimination of ceftazidime? Rev Infect Dis. 1984 Sep-Oct;6(5):732–735. doi: 10.1093/clinids/6.5.732. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Burman L. A., Linderholm H., Trollfors B. Ceftazidime: pharmacokinetics in patients and effects on the renal function. J Antimicrob Chemother. 1982 Sep;10(3):199–206. doi: 10.1093/jac/10.3.199. [DOI] [PubMed] [Google Scholar]
- Swabb E. A., Sugerman A. A., McKinstry D. N. Multiple-dose pharmacokinetics of the monobactam azthreonam (SQ 26,776) in healthy subjects. Antimicrob Agents Chemother. 1983 Jan;23(1):125–132. doi: 10.1128/aac.23.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welage L. S., Schultz R. W., Schentag J. J. Pharmacokinetics of ceftazidime in patients with renal insufficiency. Antimicrob Agents Chemother. 1984 Feb;25(2):201–204. doi: 10.1128/aac.25.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]


