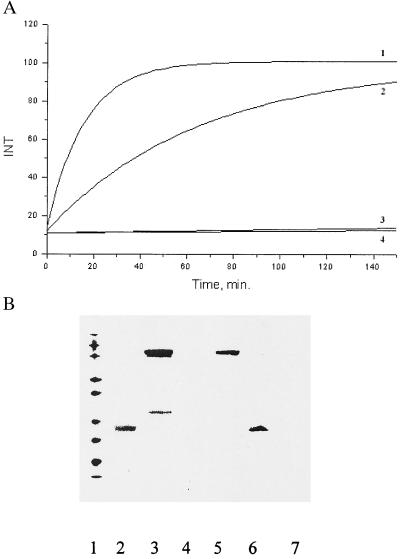
Figure 2.
Modifications of cholinesterase enzymes and antiidiotypic mAb by MCA-phosphonate. (A) Kinetics of interaction of MCA-phosphonate (compound I) with BtChoEase (1) and AcChoE (3) enzymes and with 9A8 (2) and IgM 2H11 (4) antibodies. Assays were performed on an Aminco spectrofluorimeter (excitation, 365 nm, and emission, 450 nm). Protein concentration was 55 nM, and substrate concentration was 1 μM in PBS, pH 7.8. (B) Modification of 9A8 mAb H chain by compound II. Protein concentration was 5.5 pM, and substrate concentration was 100 pM in PBS, pH 7.8. Western blot shown was stained by neutravidin-HRP conjugate. Lanes: 1, 10-kDa molecular mass markers; 2, trypsin modified by biotinylated 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF); 3, 9A8 modified by biotinylated AEBSF; 4, monoclonal IgM 2H11 modified by biotinylated AEBSF; 5, 9A8 modified by compound II; 6, trypsin modified by compound II; 7, monoclonal IgM 2H11 modified by compound II.