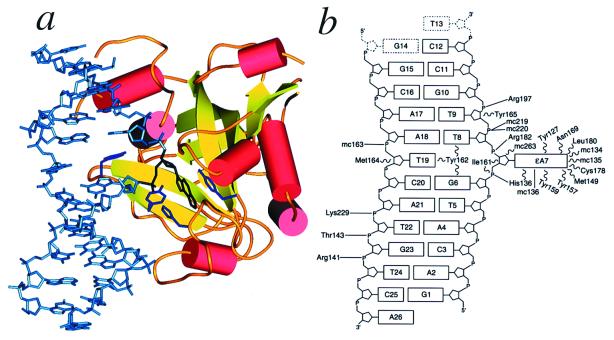
Figure 1.
Crystal structure of the E125Q AAG/ɛA-DNA complex. (a) The ɛA base (black) is flipped into the protein active site to stack between Tyr-127 on one side and His-136 and Tyr-159 on the other (shown in purple). Tyr-162 intercalates between the bases that flank the flipped-out ɛA, filling the abasic gap in the DNA. (b) Schematic diagram of contacts between AAG and the ɛA–DNA. The flipped-out ɛA base (labeled ɛA7) participates in a hydrogen-bonding interaction with the main chain amide of His-136 (solid line labeled “mc136”) and many van der Waals interactions (wavy lines) with residues of the active site (see Fig. 4). Hydrogen bonds and salt bridges (solid lines) with the DNA backbone anchor the protein to DNA. The nucleotides T13 and G14 (dashed outlines) are not visible in the electron density.