FIGURE 3.
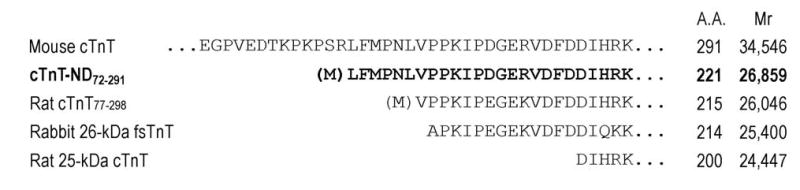
NH2-terminal truncations of cTnT. NH2-terminal amino acid sequencing of the cTnT fragment revealed a single truncation site between residues Arg71 and Leu72. The Met in bracket indicates the addition of an initiation codon for expressing cTnT-ND72-291 in E. coli (Figure 4). Amino acid sequence alignment demonstrated that the cTnT NH2-terminal truncation removes the entire variable region (Figure 5), similar to the NH2-terminal truncated fast TnT previously isolated from rabbit skeletal muscle (rabbit 26-kDa fsTnT, 32) and a model protein previously studied (Rat cTnT77-298, 16). In contrast, a caspase cleavage-produced cTnT fragment (Rat 25-kDa cTnT, 29) involves the deletion of a part of the conserved region. The number of amino acids (A.A.) and calculated molecular weights of these proteins are indicated after the sequences.
