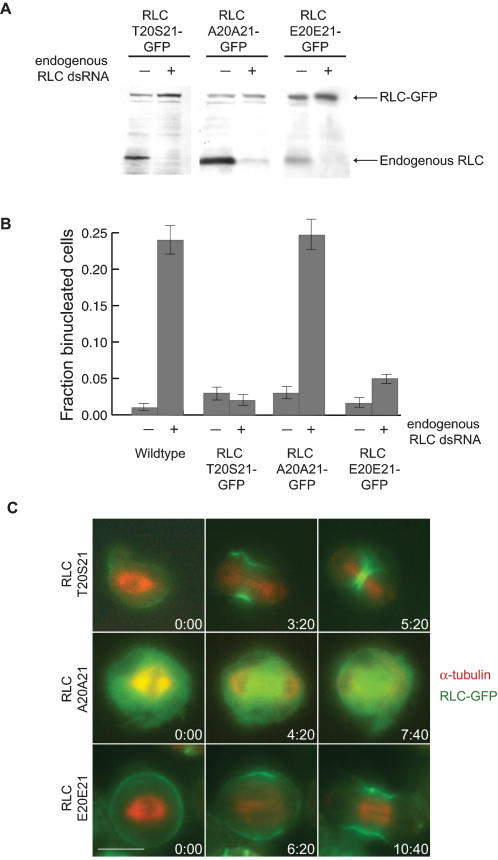
Figure 2. Phosphorylation of the RLC is necessary for cytokinesis and myosin II recruitment to the cleavage furrow.
(A) Expression of mutant RLC constructs with alternative codons allows for depletion of only the endogenous RLC by RNAi. Cells expressing RLC-GFP constructs were treated with dsRNA that targets the endogenous sequence for 5 days. Proteins in the cell lysates of treated and untreated cells were separated by SDS-PAGE and immunoblotting with an anti-RLC antibody revealed that the lower molecular weight endogenous RLC is specifically depleted while the higher molecular weight GFP chimera is still expressed. (B) Phosphorylation of the RLC is essential for cytokinesis. S2 cells were treated with dsRNAs for 5 days to deplete the endogenous RLC and the fraction of approximately 100 cells that were binucleated (mean±SE) as measured by DAPI staining was calculated. The bar graphs show fraction of binucleate cells with and without treatment with RNAi against native RLC for wild type cells, cells expressing wildtype RLCT20S21-GFP, non-phosphorylatable RLCA20A21-GFP, and phospho-mimic RLCE20E21-GFP. (C) Phosphorylation of the RLC is essential for myosin II recruitment to the cleavage furrow. S2 cells expressing RLC-GFP chimeras (green) and RFP-α-tubulin (red) were depleted of the endogenous RLC by RNAi treatment for five days, and timelapse fluorescence microscopy was used to film cells as they attempted to divide. Both RLCT20S21-GFP (Row 1) and RLCE20E21-GFP (Row 3) were recruited to the equatorial cortex during mitosis and the cells contracted. However, RLCA20A21-GFP (Row 2) was not recruited to the equatorial cortex at anaphase and the cells did not contract. The scale bar = 5 µm.