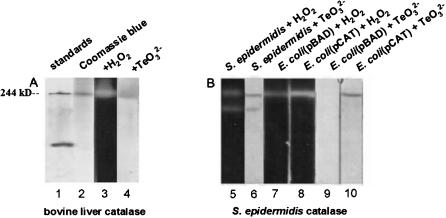
Figure 3. Catalases have tellurite reductase activity in situ.
Assays for the H2O2 dismutase and TeO3 2− reductase activities of catalase in situ were carried out by resolving proteins on polyacrylamide gels, incubating gel strips in the presence of substrate, and developing gel strips to reveal the presence of specific products of each reaction (Woodbury et al. 1971; Gregory and Fridovich 1974).
For dismutase assays, 25 µg of protein were loaded in each gel lane; 250 µg of protein was used for tellurite reductase assays.
(A) The results in Fig. 3A show that bovine liver catalase (a tetramer of 255 kDa) migrates as a single band on native gels (lane 2) with an apparent molecular mass similar to that of a 244 kD standard (lane 1), and has both hydrogen peroxide dismutase (lane 3) and tellurite reductase (lane 4) activities.
(B) The activities of catalase present in extracts made from E. coli Top10 cells carrying the plasmid vector pBAD display lower H2O2 dismutase and TeO3 2− reductase activities (lanes 7 and 9) than does an otherwise isogenic strain with plasmid pCAT, which expresses the S. epidermidis CH katA (catalase) gene (lanes 8 and 10); these activities most likely correspond to the predominant tetrameric form of catalase.
In crude extracts of S. epidermidis, two different bands of each activity are observed (lanes 5 and 6).