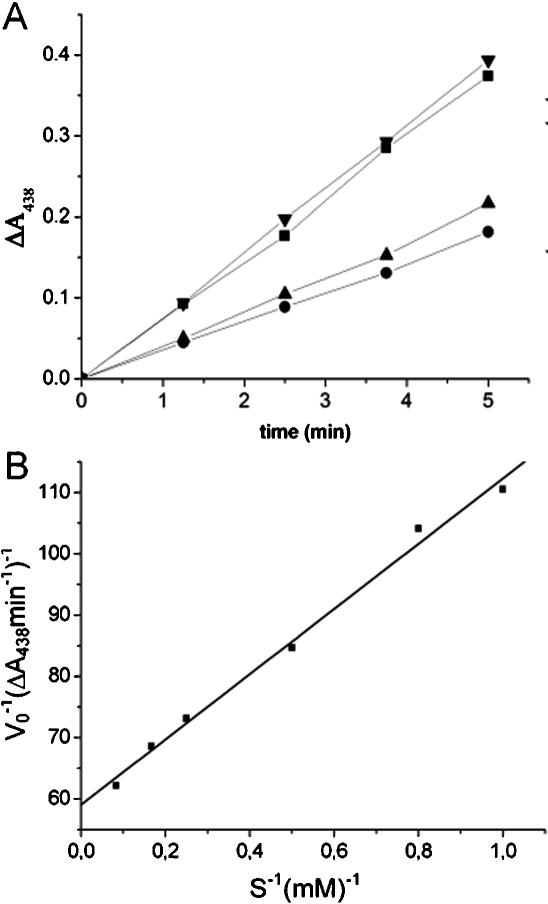
Figure 5. The reduction of tellurite by bovine liver catalase produces superoxide anion and obeys simple Michaelis-Menten kinetics.
In these experiments, we measure the rate of evolution of superoxide radical as the product of the tellurite reductase reaction mediated by catalase, by the ability of superoxide to reduce the tetrazolium derivative WST-1.
(A) The rates of change in absorbance at 438 nm in standard reactions with 5 mM K2TeO3 were measured.
Reactions with tellurite (◂;complete) show a higher rate of WST-1 reduction than do reactions without substrate (•; - K2TeO3).
Without substrate, WST-1 is reduced at a lower, background rate by NADPH.
When superoxide dismutase is added to the reaction (▴; +SOD), only the lower rate of reduction is observed; in contrast, the addition of β-amylase (▪; +AMY) has little effect on the rate of reduction, demonstrating that the most abundant reductant of WST-1 in these assays is superoxide radical.
(B) The reciprocals of the initial velocities of tellurite reduction, measured as the changes in absorbance at 438 nm min−1 due to the coupled reduction of WST-1, plotted versus the reciprocals of the concentrations of the substrate (S), potassium tellurite, (mM) for reactions carried out under standard conditions (see Materials and Methods for details).