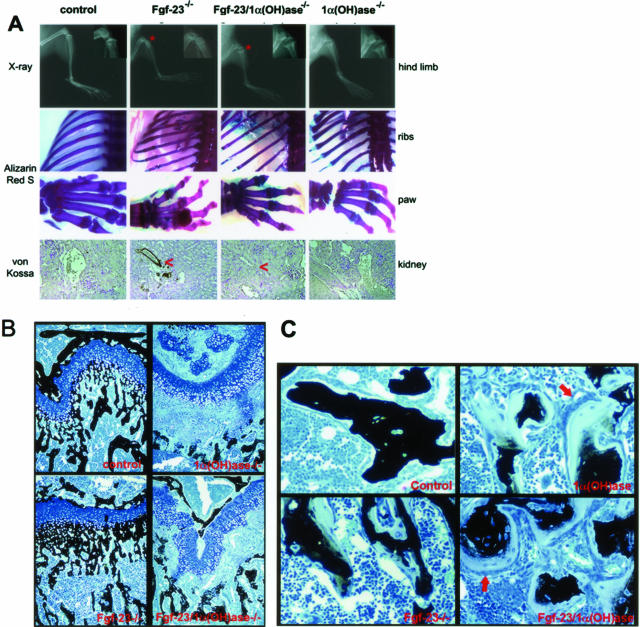
Figure 4.
A: Top: X-ray autoradiography of hindlimbs from a control, Fgf-23−/−, Fgf-23−/−/1α(OH)ase−/−, and 1α(OH)ase−/− mouse at 6 weeks. Asterisk depicts the ricketic widening of the growth plate in double-mutant versus Fgf-23−/− mice. Middle: Alizarin Red S-stained skeletal elements (ribs and paws) from each animal demonstrate loss of nodule formation in Fgf23−/−/1α(OH)ase−/− mutants. Bottom: Von Kossa staining of kidneys of each genotype. Red arrowheads point to the calcification of renal vessels of Fgf-23−/−, which is completely eliminated in Fgf-23−/−/1α(OH)ase−/− double mutants. B: Three-μm-thick undecalcified sections from 6-week-old control, Fgf-23−/−, Fgf-23−/−/1α(OH)ase−/−, and 1α(OH)ase−/− bones were stained with von Kossa/McNeal. Black staining represents mineralization. More mineral deposition is found in the area below the growth plate (methaphysis) in Fgf-23−/− mice. In contrast, large areas of unmineralized osteoid (light blue) are found in bones of Fgf-23−/−/1α(OH)ase−/− and 1α(OH)-ase−/− mice. C: Cancellous bone of 1α(OH)ase−/− and Fgf-23−/−/1α(OH)ase−/− compound mutants show hyperactive, cuboidal osteoblasts on top of extremely thick osteoid layers (red arrows). Osteoblasts in Fgf-23−/− mice appeared more flat, and osteoid seams were thinner. Original magnifications, ×20 (A, B).