Figure 3. Crystal structures of the proteasome 20S surface.
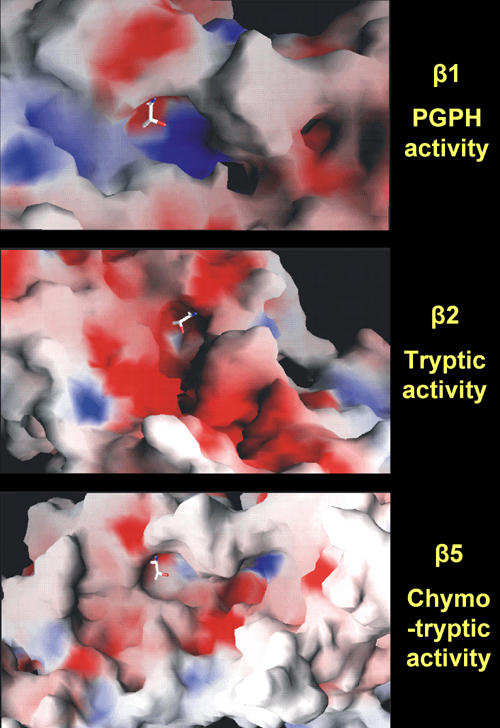
The images represent the crystal structures of 20S proteasome surfaces. Cleavage of peptides takes place at active sites of the β-subunits after acidic (peptidylglutamyl peptide hydrolyzing (PGPH) activity in β1), basic (trypsin-like activity in β2), and hydrophobic (chymotrypsin-like activity in β5) residues. Each image shows the nucleophilic Th1 in sticks, the basic residues in blue, the acidic residues in red, and the hydrophobic residues in white. Image courtesy by Olivier Coux, CRBM-CNRS, France.
