Figure 1.
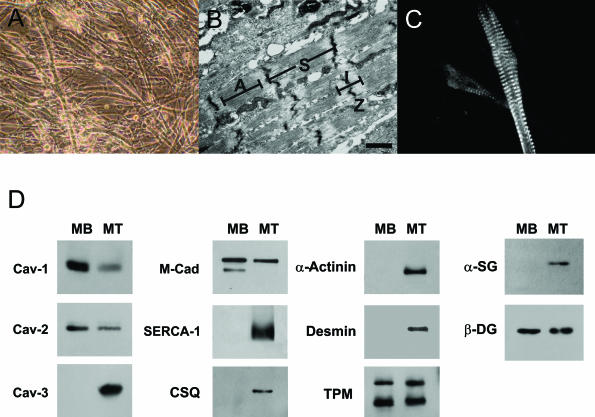
Cav-1 and Cav-2 are down-regulated during skeletal muscle differentiation and myotube formation in primary cultures of INK4a(−/−) skeletal muscle myoblasts. To directly evaluate Cav-1 and Cav-2 expression levels during skeletal muscle differentiation, we derived and characterized a novel INK4a(−/−) skeletal muscle cell line. A: INK4a(−/−) skeletal muscle myoblasts undergo spontaneous differentiation and form myotubes approximately 2 to 3 days after they reach ∼100% confluence. A phase-contrast image of these differentiated myotubes is shown. B: Ultrastructural analysis of INK4a (−/−) myotubes by transmission electron microscopy. Note the presence of sarcomeres (S). Z, Z-line; A, A-band; I, I-band. C: Myotubes were immunostained with an antibody that recognizes the sarcomeric myosin heavy chain of vertebrate muscle (mAb MF20). Note the striated pattern, characteristic of differentiated skeletal muscle myotubes. D: INK4a(−/−) myoblasts undergo differentiation and express muscle-specific marker proteins. Note that Cav-1, Cav-2, and M-cadherin are predominantly expressed in the undifferentiated cells, with their expression levels decreasing during differentiation. Conversely, markers of differentiated skeletal muscle cells, such as Cav-3, SERCA-1, calsequestrin (CSQ), α-actinin, desmin, and α-sarcoglycan (α-SG), are elevated during differentiation. Significantly, other proteins seem to be expressed equally in undifferentiated and differentiated INK4a(−/−) skeletal muscle cells, namely tropomyosin (TPM) and β-dystroglycan (β-DG). Each lane contains an equal amount of total protein. MB, myoblasts (undifferentiated); MT, myotubes (differentiated). Scale bar = 1 μm.