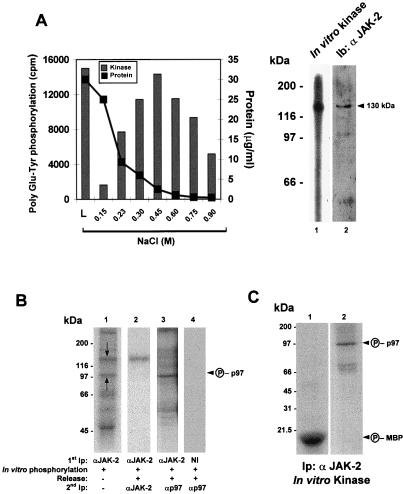
Figure 2.
Purification of tyrosine kinase activities from LDM. (A) Low-density microsomes from rat liver (600 μg to 5 mg) solubilized were incubated with ATP-agarose beads. Eluted fractions were then tested for the presence of kinase activity by incubation with 0.5 mg/ml poly Glu-Tyr, and radioactivity was measured by scintillation counting. Protein concentration was measured for each fraction and is reported on the graph. L represents 5% of total protein lysate chromatographed on ATP-agarose. Fractions containing the highest specific activity were concentrated and either in vitro autophosphorylated (Right, lane 1) or directly resolved by SDS/PAGE followed by immunoblotting with anti-JAK-2 antibodies (Right, lane 2). (B) In vitro phosphorylation of JAK-2 immunoprecipitate from LDM (lane 1, overnight exposure) or in vitro phosphorylation of JAK-2 immunoprecipitate from LDM, followed by release and sequential immunoprecipitation with anti-JAK-2 antibodies (lane 2, overnight exposure) or anti-p97 antibodies (lane 3, 40-h exposure). In lane 4, a nonimmune serum (NI) was used in the primary immunoprecipitation, and an anti-p97 was used for the secondary immunoprecipitation (overnight exposure). (C) In vitro phosphorylation of 10 μg of myelin basic protein (MBP, lane 1) or purified p97 (lane 2) by JAK-2 immunocomplex from LDM. A representative experiment is shown (n = 2).