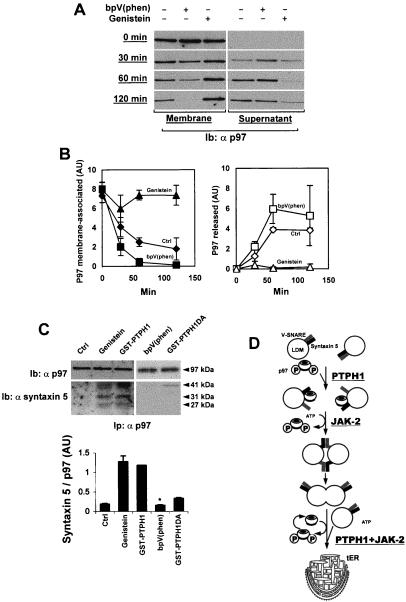
Figure 5.
(A) Association of p97 to the membranes is regulated by its tyrosine phosphorylation. LDM were incubated as previously described (10–12) in the presence or absence of 100 μM bpV(phen) or 350 μM genistein for different periods. The samples were then placed on ice and received 1 ml each of 0.25 M sucrose containing 3 mM imidazole. The samples were then centrifuged at 100,000 × g for 30 min. The proteins in the membrane pellets were dissolved directly in Laemmli buffer. The proteins in the supernatant fractions were concentrated by trichloroacetic acid precipitation. Proteins were then separated by SDS/PAGE, followed by immunoblotting with anti-p97 antibodies. (B) Densitometric analysis of the above immunoblots. Each value is the mean of three independent experiments ± SD. Closed symbols stand for membrane-associated p97 and open symbols for p97 released from the membranes, respectively, ♦,⋄ CTL; ▴,▵, genistein; ■,□, bpV(phen). (C) LDM (600 μg) were first incubated 45 min on ice as previously described, except that Hepes was replaced by 6 mM imidazole, pH 7.4; this incubation buffer contained or not 350 μM genistein or 30 μg of GST-PTPH1 or GST-PTPH1D811A or 100 μM bpV(phen). Microsomes were then solubilized in the same buffer containing 1.5% CHAPS. After spinning in a microfuge for 15 min at maximal speed, lysates were immunoprecipitated with anti-p97 antibodies. Immunoprecipitates were then resolved by SDS/PAGE, transferred onto nitrocellulose, and immunoblotted with either anti-syntaxin 5 antibodies or anti-p97 antibodies and revealed by ECL. A representative experiment is shown (control, genistein, bpV(phen), and GST-PTPH1DA, n = 3; GST-PTPH1, n = 2). Quantitation was made by scanning densitometry of the x-ray films and represents the mean ± SD (when n = 3); * indicates significant values P < 0.04. For syntaxin 5, the intensities corresponding to the three bands visualized were added. (D) Representative scheme of the sequence of events regulating p97 association with LDM.