Abstract
Methods: Thirty children (mean 4.3 (SD 0.3) years) received 200 µg budesonide twice daily by NC or AC, both with the mask provided, in a randomised, two month crossover trial. Twenty four hour urinary free cortisol (UFC) was determined as a measure of HPA suppression.
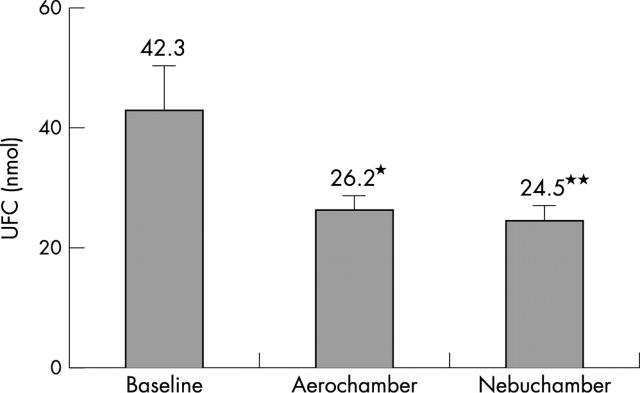
Results: UFC decreased from 42.3 (7.8) nmol UFC/nmol creatinine control to 26.2 (2.4) (p = 0.06 v control) after AC, and to 24.5 (2.5) (p = 0.04 v control) after NC (p = 0.4 AC v NC).
Conclusions: Despite a greater total dose delivered to the mouth, NC is not associated with greater HPA suppression when using 400 µg/day budesonide under real life conditions in young children.
Full Text
The Full Text of this article is available as a PDF (70.9 KB).
Figure 1.
Urinary free cortisol (corrected for creatinine secretion) at baseline and after each treatment period (mean (SD)). *p = 0.06 v baseline; **p = 0.04 v baseline.