Abstract
Angiostatic therapies designed to inhibit neovascularization associated with multiple pathological conditions have only been partially successful; complete inhibition has not been achieved. We demonstrate synergistic effects of combining angiostatic molecules that target distinct aspects of the angiogenic process, resulting in the complete inhibition of neovascular growth associated with development, ischemic retinopathy, and tumor growth, with little or no effect on normal, mature tissue vasculature. Tumor vascular obliteration using combination angiostatic therapy was associated with reduced tumor mass and increased survival in a rat 9L gliosarcoma model, whereas individual monotherapies were ineffective. Significant compensatory up-regulation of several proangiogenic factors was observed after treatment with a single angiostatic agent. In contrast, treatment with combination angiostatic therapy significantly reduced compensatory up-regulation. Therapies that combine angiostatic molecules targeting multiple, distinct aspects of the angiogenic process may represent a previously uncharacterized paradigm for the treatment of many devastating diseases with associated pathological neovascularization.
Keywords: combination therapy, eye disease, tumor therapy, neovascularization
Neovascularization contributes to the pathogenesis of tumor growth (1) and metastasis (2) as well as the vast majority of diseases that lead to catastrophic loss of vision (3–5). Largely as a result of an increased understanding of mechanisms underlying angiogenesis, a large number of angiostatic molecules have been described (6), many proving to be valuable clinical adjuncts to conventional chemotherapy, reducing tumor loads and prolonging survival. Angiostatics have also proven to be modestly effective therapeutics for neovascular eye diseases, reducing the rate of severe vision loss (7). However, treatments using single angiostatics have yet to demonstrate complete inhibition of neovascular growth in the clinic and thus far have only delayed tumor growth (8, 9) or vision loss (5).
Angiogenesis, the growth of new blood vessels from preexisting capillaries, is a fundamental biological process essential to survival of the organism. As such, redundant mechanisms have evolved to facilitate new blood vessel growth, and, in vivo, angiogenesis is likely to be initiated by the combined activation of multiple pathways. These compensatory mechanisms may be what ultimately limit the therapeutic potential of antiangiogenic monotherapies, because blocking a single pathway may induce compensation by other proangiogenic pathways (10). During the angiogenic process, endothelial cell proliferation and migration is first stimulated by multiple growth factors (1). Subsequently, dividing endothelial cells mediate controlled degradation of the extracellular matrix (ECM) (11), navigate the extracellular milieu by using various ECM receptors and cell-cell adhesion molecules (12, 13), organize the formation of a central lumen, and mature into a functional vessel.
We hypothesized that combining antiangiogenic treatments may yield higher efficacy than monotherapy. Therefore, the combined action of three classes of angiostatic compounds, each targeting different aspects of the angiogenic process, was tested. To block stimulation, we used a VEGF aptamer chemically identical to Macugen, recently approved for the treatment of neovascular eye diseases (14). To target extracellular matrix-mediated endothelial cell survival, we used a small-molecule αvβ3 and αvβ5 integrin antagonist (EMD472523, Merck, Darmstadt, Germany) (15, 16). To block endothelial intracellular adhesion and lumen formation, we used T2-TrpRS (T2), a proteolytic fragment of tryptophan tRNA synthetase with angiostatic activity linked to its ability to block VE-cadherin-mediated adhesion. Although the precise mechanism of action of T2 is not defined, it is important to note that it does not bind VEGF, VEGF receptors, or αvβ3 and αvβ5 integrins (refs. 17 and 18 and M.I.D. and M.F., unpublished observations). Here, we demonstrate profound synergistic antiangiogenic activity by combining antiangiogenics that target distinct aspects of the angiogenic process and suggest that such combination therapy may be effective in the treatment of neovascular diseases. We also observe that the synergistic effects of combination therapy may be due to blocking up-regulation of compensatory pathways.
Results
Combination Therapy Enhances Angiostatic Activity During Development.
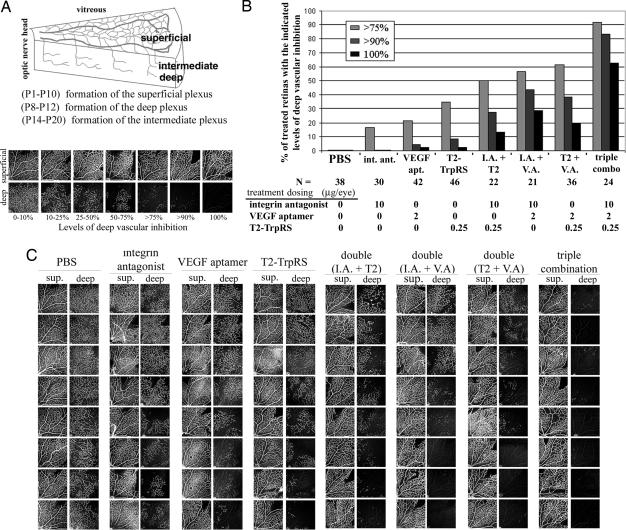
The neonatal mouse retinal developmental angiogenesis model was used to test the efficacy of each monotherapy (Fig. 1A). Optimal effective doses of 2.0 μg per eye (215 pmol) for the VEGF aptamer, 10 μg per eye (20 nmol) for the integrin antagonist, and 0.25 μg per eye (5.2 pmol) for T2, were found. At these maximum effective doses, monotherapy treatment resulted in no angiostatic effect in ≈1/3 of the treated retinas and caused high levels of inhibition (>75%) in 17–35% of the retinas, depending on the monotherapy tested (Fig. 1 B and C). In combination, the angiostatic effect was markedly improved, with the combination of all three (1× triple) being better than the combination of any two. Only 2 of the 24 retinas treated with triple combination demonstrated any substantial neovascularization, whereas 20 had >90% inhibition, of which 15 (63%) exhibited complete inhibition of deep vascular plexus formation where no neovascular sprouts were observed (Fig. 1 B and C). This result is a striking improvement over angiostatic monotherapies, which resulted in complete inhibition in only 2 of 118 (2%) retinas. Central vessels of the superficial plexus that had formed before injection, as well as retinal morphology, remained normal, indicating no detectable toxicity to established vasculature (Fig. 1C).
Fig. 1.
Combination therapy enhances angiostasis in a neonatal eye model. (A) During the first three postnatal weeks, the mouse retinal vasculature forms three distinct planar plexuses. At postnatal day (P)8, vessels in the superficial plexus branch and form a deep plexus. To assess angiostasis, intravitreal injections were performed at P7, and inhibition of the deep vessels was scored 5 days later. Neural retina and previously formed superficial plexus were evaluated for toxicity. (B) Combination angiostatic therapy dramatically increases the percentage of treated retinas with high levels of neovascular inhibition. (C) Images from a representative experiment directly comparing angiostatic monotherapy and combination therapy at optimal doses (1×). sup., superficial; I.A., integrin antagonist; V.A., VEGF aptamer.
Combining Angiostatic Therapy Results in Synergistic Activity.
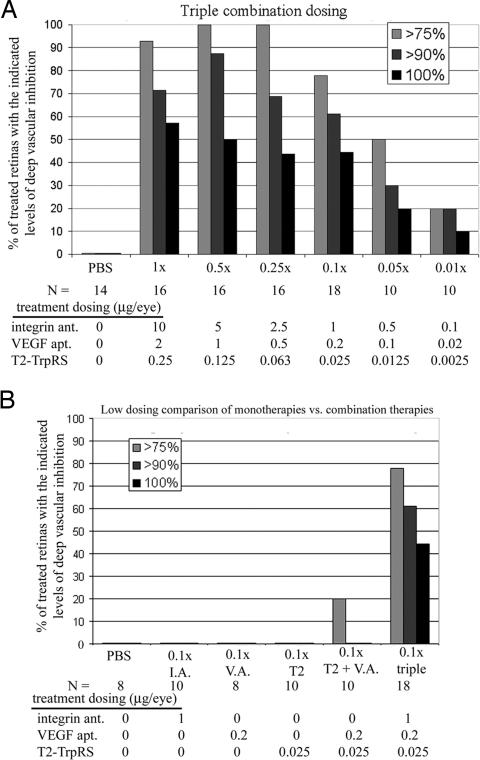
In combination, the angiostatic therapies were effective at much lower concentrations than when used as individual monotherapies. Even when diluted up to 100-fold (0.01×), triple combination inhibited angiogenesis at levels comparable to optimal (1×) doses of any monotherapy (Fig. 2A). Ten-fold dilution of the triple combination (0.1× triple) demonstrated extensive neovascular inhibition with complete inhibition observed in 44% of the treated retinas. At these same 0.1× concentrations, the angiostatic activity of each monotherapy was negligible (Fig. 2B), demonstrating that combining multiple angiostatic drugs was synergistic rather than additive. It is important to note that, during injection, reflux of the drug can be an issue because of injection of small volumes (0.5 μl) into small neonatal mouse eyes. This is a potential source of the variability observed with monotherapy treatments at optimal doses; any significant leakage of the drug could result in ineffective concentrations. In contrast, combination therapy, which is effective at much lower concentrations because of synergism, would remain effective despite any substantial reflux that may occur during individual injections.
Fig. 2.
Combination angiostatic therapy is synergistic. (A) Triple combination therapy is potent at highly diluted concentrations. (B) Doses that exhibit minimal angiostatic activity as monotherapies (0.1× optimal dose) still exhibit potent angiostatic activity when used in combination. I.A., integrin antagonist; V.A. VEGF aptamer; ant., antagonist; apt., aptamer.
Similar synergistic properties were observed when the VEGF aptamer was replaced by commercially available anti-VEGF products such as Macugen, a specific inhibitor of VEGF165 (19), or Avastin, an anti-VEGF antibody that blocks all VEGF-A isoforms and is currently approved for the treatment of metastatic colorectal cancer (20). Although Avastin has low reactivity with mouse VEGF compared with human VEGF, Avastin monotherapy demonstrated significant activity similar to the VEGF aptamer, and synergism with T2 and the integrin antagonist were still apparent. Combining three VEGF antagonists did not result in improved angiostatic activity as compared with VEGF monotherapy alone [supporting information (SI) Fig. 6], suggesting that combining angiostatics with distinct targets is important for synergistic activity. For an inclusive table of the developmental model data, see SI Table 1.
Combination Therapy Inhibits Pathological Neovascularization.
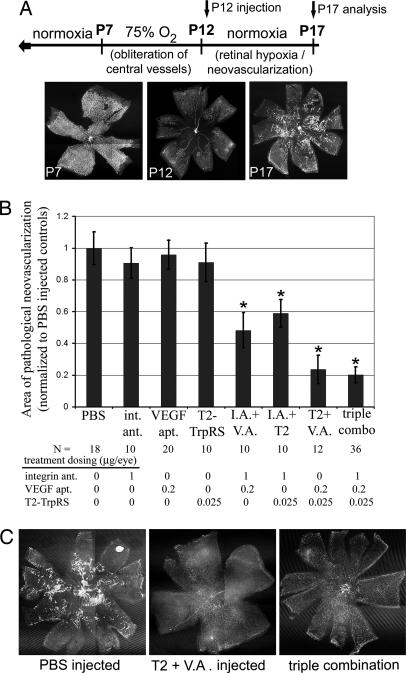
To study the synergistic properties of combination therapy in a model of pathological angiogenesis, we used the mouse model of oxygen-induced retinopathy (OIR), a commonly used model of hypoxia-induced pathological neovascularization with consistent, quantifiable vascular changes (Fig. 3A) (21, 22). Retinas treated with the combination of any two angiostatics displayed significantly less pathological neovascularization than retinas treated with the respective monotherapies at the same concentration (Fig. 3B). The most successful combination was T2 plus VEGF aptamer, which resulted in an average 80% reduction of pathological neovascularization compared with PBS-treated retinas, with many of the treated retinas displaying no pathological neovascularization at all (Fig. 3C). In the OIR model, triple-combination therapy did not offer significant improvement of angiostatic activity beyond that observed with this double combination.
Fig. 3.
Combination angiostatic therapy inhibits ischemia-induced, pathological neovascularization. (A) In the mouse OIR model, hyperoxia (75% oxygen from P7 to P12) results in vascular obliteration. When mice are returned to normoxia (P12), retinas becomes hypoxic because of a lack of vessels, resulting in pathological neovascularization (P17). (B) Combination therapies significantly inhibit pathological neovascularization compared with vehicle control and angiostatic monotherapies (asterisks indicate P values <0.01 vs. PBS and each monotherapy, error bars represent SEM). (C) Many retinas treated with T2/VEGF aptamer combination (Center), or triple combination (Right) demonstrated nearly complete inhibition of neovascular tuft formation, seen as brightly stained areas in the PBS control-treated retinas (Left). int., integrin; ant., antagonist; apt., aptamer; I.A., integrin antagonist; V.A., VEGF aptamer.
Combination Therapy Results in Obliteration of Tumor Vasculature.
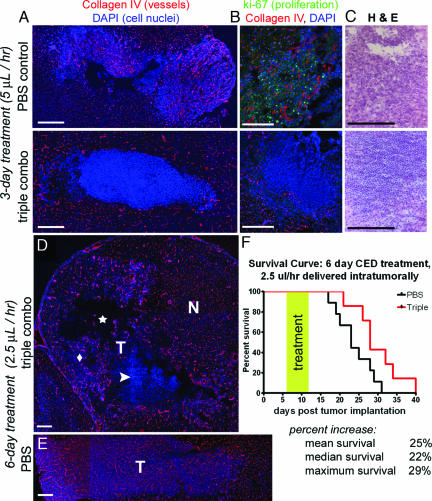
We next tested the effects of triple-combination therapy in a 9L rat gliosarcoma model. This is a rapidly growing, highly vascular tumor model in which intra-cerebral 9L tumors are established in adult rats, uniformly leading to tumor-related mortality 3–4 weeks after implantation (23). Rats were treated with either PBS or a combination of T2 (1.5 mg/ml), VEGF aptamer (2.0 mg/ml), and integrin antagonist (5 mg/ml) via intratumoral convection-enhanced delivery, initiated 6 days after tumor implantation. After 3-day delivery of triple-combination therapy (5 μl/hr), large avascular tumor regions were found (Fig. 4 A and B), associated with a massive influx of mononuclear cells (Fig. 4C). Four of seven rats had nearly complete vascular obliteration in the tumor region, whereas three had regions of normally vascularized tumor growing peripheral to the avascular zone. After 6 days of treatment using a slower convection rate (2.5 μl/hr), large avascular areas and mononuclear cell infiltrate were associated with significantly reduced tumor; empty cavities were observed where tumor would normally be located (Fig. 4D). Small areas of tumor with normal vasculature were often observed in adjacent regions near the periphery, presumably at the outer limits of effective drug convection, demonstrating a correlation between vascular integrity and tumor persistence. Normal brain vasculature was not affected. Tumors in PBS-treated rats displayed typical tumor vascularization (Fig. 4 A and E). A much smaller infiltrate of mononuclear cells, which may be the result of an immune response created by the delivery technique or naturally occurring necrosis at the tumor center, was also observed in the PBS-treated tumor regions (Fig. 4C). Monotherapy treatment resulted in no significant difference in tumor vascularization compared with PBS treatment (SI Fig. 7). In three separate survival studies, treatment with triple-combination therapy significantly prolonged rat survival. When treated with two separate 24-hour pumps (8 μl/hr) at days 6 and 13 after tumor implantation, median survival rates were increased by 18%. When treated with 6 days of continuous infusion (2.5 μl/hr) starting at 6 days after tumor implantation, mean survival was increased by 25% and maximum survival by 29% (Fig. 4F).
Fig. 4.
Combination angiostatic therapy obliterates tumor vasculature, decreases tumor size, and increases survival. (A) Vessels are absent in tumors of animals treated for 3 days with triple combination therapy (Lower). Tumor vasculature is normal in control PBS treated tumors (Upper). (B) PBS treated tumors are highly proliferative (Upper) as indicated by ki-67 staining, whereas no proliferation is seen in the avascular triple combination-treated tumors (Lower). (C) Massive infiltrates of mononuclear cells are observed in avascular tumor regions after treatment with triple combination (Lower). In PBS controls (Upper) small areas of mononuclear cell infiltration are observed within large areas of normal tumor growth. (D) After 6 days of triple combination treatment, empty cavities (star), areas of mononuclear infiltrate (arrowhead), and smaller areas of reduced vasculature (diamond) are all observed within the tumor implantation areas (T). Normal brain vasculature in adjacent regions is not affected (N). (E) Rats with PBS-treated tumors have extensive, highly vascular tumors. (F) Triple combination significantly increases survival. Treatments were initiated 6 days after tumor implantation by using constant, local, convection-enhanced delivery to the central tumor. (Scale bars: 0.5 mm.)
Compensatory Up-Regulation Follows Angiostatic Monotherapy Treatment.
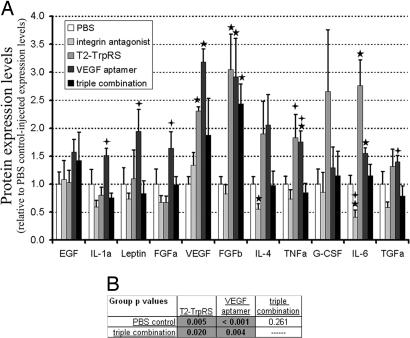
To test the theory that angiostatic monotherapies induce compensatory up-regulation of proangiogenic factors, an ELISA-based assay was used to quantify the expression levels of angiogenic proteins in normal (nontreated) retinas and in retinas treated with vehicle (PBS), angiostatic monotherapies, or combination therapy. Significant up-regulation of VEGF, basic FGF (FGFb), and IL-6 was observed in retinas treated with either T2 or VEGF aptamer monotherapy solutions (Fig. 5A). Only FGF-β remained significantly up-regulated in triple-combination-treated retinas. Of 11 proangiogenic factors that were expressed in the five retinal groups, many were consistently up-regulated after T2 or VEGF aptamer monotherapy. Overall changes in the expression of proangiogenic factors demonstrated significant up-regulation after treatment with T2 (P < 0.01) or the VEGF aptamer (P < 0.001), but not in the triple combination-treated retinas (P = 0.26), compared with PBS-treated retinas (Fig. 5B). Overall expression levels of proangiogenic factors were also significantly lower in retinas treated with triple combination compared with those treated with T2 or VEGF aptamer monotherapies, demonstrating global reduction in the levels of compensatory up-regulation. Our observation that multiple angiogenic pathways are up-regulated in response to monotherapy, but not to triple combination treatment, supports the hypothesis that compensatory mechanisms might prevent single angiostatics from inhibiting neovascularization.
Fig. 5.
Angiostatic monotherapy results in compensatory up-regulation of proangiogenic factors; combination therapy reduces such up-regulation. (A) Solutions consisting of PBS, monotherapies, or triple combination angiostatic therapy were injected into developing mouse eyes (P7) and protein expression levels of angiogenic factors were analyzed by an ELISA-based assay (P11). Stars and crosses indicate individual P values <0.05 compared with PBS-injected controls, or triple combination-treated retinas, respectively. (B) Statistical analyses of overall protein expression changes demonstrate that T2 and VEGF aptamer monotherapy treatments resulted in significant global increases in proangiogenic factor expression compared with PBS control-treated and triple combination-treated retinas.
Discussion
We have demonstrated dramatic, synergistic angiostatic activity by combining compounds that inhibit distinct aspects of the angiogenic process. Use of triple-combination angiostatic therapy resulted in nearly complete inhibition of developmental and pathological neovascularization as well as substantial reduction in tumor-associated vascular growth. In each case, normal, established vasculature was not affected. In addition, combined angiostatic therapy resulted in reduced tumor growth and significantly prolonged the lives of tumor-bearing rats. Combination therapies that target other pathways may be equally, or even more, effective, but our data suggest that it is important for any combination angiostatic therapy to use compounds that each target distinct aspects of the angiogenic process. Combining angiostatics targeting identical mechanisms (i.e., multiple VEGF antagonists) did not result in enhanced antiangiogenic activity (SI Fig. 6). These results provide proof of concept that targeting multiple angiogenic pathways can increase the effectiveness of antiangiogenic therapy and may provide an option for the treatment of neovascular diseases where complete inhibition of neovascularization is desirable.
Significant compensatory up-regulation of various proangiogenic factors was demonstrated after treatment with T2 or the VEGF aptamer. This result supports the concept that persistent angiogenesis after angiostatic monotherapy treatment at least partially results from up-regulation of compensatory angiogenic pathways. The fact that compensatory up-regulation was observed in normal, developing (nontumor) tissues raises the interesting question of how stable populations of vascular-related cells detect the loss of activity of one angiogenic factor and subsequently activate alternative pathways. Up-regulation was significantly reduced after treatment with combination therapy, suggesting that combination therapy prevents natural compensation, resulting in enhanced angiostasis. Significant up-regulation was not observed after treatment with the integrin antagonist. However, being the least potent of the angiostatics tested (Fig. 1B), up-regulation may be below the detection sensitivity of our techniques. Alternatively, compensatory mechanisms involving the up-regulation of other survival and antiapoptotic factors, such as P53 rather than angiogenic stimuli, have been described during antagonism of α-v integrins (24).
Although combination therapy significantly reduces up-regulation of proangiogenic factors compared with VEGF antagonism or T2 monotherapy, some level of up-regulation is still evident. Many studies have demonstrated that angiogenesis is stimulated by an increase in proangiogenic factors beyond a critical point, a process commonly called the angiogenic switch (25). The observed decreases in expression of various proangiogenic factors may lower the angiogenic stimuli below this critical threshold, resulting in substantially reduced neovascularization. However, it is also likely that combination angiostatic therapy prevents angiogenesis by blocking multiple pathways that are either inherent or up-regulated because of angiostatic treatment. Downstream angiogenic signaling pathways overlap substantially (6). This highly integrated nature of angiogenesis signaling suggests that compensatory up-regulation of proangiogenic factors, as well as the proangiogenic effects of upstream initiators, can be reduced or eliminated by blocking a critical number of downstream events.
Increasing angiostatic efficacy by combination therapy as described here has important implications for clinical use. In neovascular eye diseases, loss of vision is associated with retinal edema, bleeding, and fibrosis secondary to abnormalities in the new, dysfunctional vessels (4). Partial inhibition still leaves abnormal vessels to bleed or leak fluid, which could explain results in clinical trials where vision is initially stabilized or even improved after angiostatic monotherapy but eventually continues to decrease (26). Aside from transforming the new vessels into a normal, functional vasculature (27), the complete inhibition of abnormal vessel growth by using combination angiostatic therapy may be the best method for preventing such complications of partial treatment.
High levels of angiostasis may also be required to prevent further tumor growth and metastasis. Monotherapies that reduce, rather than eliminate, neovascular growth are only likely to reduce continued growth and metastasis of tumors (28). In fact, recent evidence suggests that much of the antitumor effects from angiostatic monotherapies may actually be the result of normalizing, rather than reducing, tumor vasculature, resulting in enhanced efficacy of other chemotherapies (29). In contrast, we have demonstrated high levels of tumor-associated vascular obliteration in an established tumor using combination angiostatic therapy. This result is associated with a significant delay in mortality in an aggressive tumor model where any significant difference between treated and control groups indicates a strong effect (23, 30). It is likely that the angiostatic compounds are cleared after treatment, leading to resumed growth in this highly aggressive tumor model. Such remaining tumor could theoretically reinitiate rapid growth, leading to eventual death. With improvements in drug delivery it may be possible to enhance survival.
Another advantage combination therapies offer is the use of relatively low doses. Elderly (e.g., age-related macular degeneration) or ischemic (e.g., diabetic) patients are likely to be collateralizing ischemic tissues, and high levels of circulating angiostatics could exacerbate or precipitate stroke or myocardial infarction (31). For these and other patients, the use of lower, but effective, doses of angiostatic therapies is desirable to minimize adverse side effects, such as inhibiting physiological neovascularization. This is particularly important when anti-VEGF strategies are used, in light of the vasculo- and neurotrophic activities associated with VEGF (32). Combination therapies that combine anti-VEGF activity with other angiostatics can maintain potent angiostatic activity at concentrations which may lessen any negative, nonangiogenic effects of VEGF antagonism. Together, our data demonstrate the potential utility of combining different angiostatic molecules for the treatment of disease-associated neovascularization. As more angiostatic molecules receive regulatory approval, their use in combination with each other should lead to more highly effective antiangiogenic therapies.
Materials and Methods
Sample Preparation.
The VEGF aptamer was synthesized as a PEG-conjugated compound (Transgenomic, Boulder, CO) chemically identical to Macugen (14, 33). Concentrations refer to active RNA aptamer rather than the total pegylated compound. Both the integrin antagonist (EMD472523; Merck) and the VEGF aptamer were stored as lyophilized powders and solubilized in RNase-free 1× PBS before use. T2 (Angiosyn, La Jolla, CA) was stored in 50% glycerol at −20°C and dialyzed into sterile 1× PBS before use. Macugen (Eyetech) and Avastin (Genentech, South San Francisco, CA) were obtained commercially. For combination therapy, individual solutions were combined at appropriate concentrations in PBS, and all treatments were applied in a single 0.5-μl intravitreal injection.
Intravitreal Injections.
All animal work adhered to strict protocol guidelines for the humane care and use of animals. Intravitreal injections were performed, retinas dissected, and the vasculature visualized as described (34). For the neonatal mouse model, intravitreal injections of 0.5-μl solutions were performed at P7 into Balb/C mice, and the resulting deep vasculature was analyzed at P12 by using anticollagen IV (AB756P; Chemicon, Temecula, CA) (Fig. 1A). OIR was induced as described by Smith et al. (21); P7 pups and their mothers were exposed to 75% oxygen for 5 days, followed by a return to room air (Fig. 3A). Intravitreal injections were performed at P12, immediately after return to normoxia. Areas of preretinal neovascular tuft formation were analyzed at P17 by using published methods (35).
Angiogenesis Array.
Intravitreal injections were performed at P7 (0.5 μl per eye), and, at P11, retinas were isolated and lysed in PBS buffer containing 1% Triton X-100 plus protease inhibitors (Roche, Indianapolis, IN). Three milligrams of total retinal lysate was hybridized to each membrane of an antibody-sandwich angiogenesis array (Panomics, Fremont, CA) according to the manufacturer's guidelines. In two separate experiments (duplicate spots = 4 replicates total), the antibody arrays were hybridized and imaged together. Expression intensities were calculated by adding the total pixel intensity for each spot. Background was subtracted by calculating the average pixel intensity for a 1-pixel ring outside the spot and subtracting this baseline value from pixel intensity values within the spot. Interarray normalization was performed by using positive-control spots (eight per array) on each array. Protein expression levels were normalized to PBS controls so that changes in protein expression could be easily assessed. For statistical analysis of treatment groups, an ANOVA t test (one-tail, equal sample variance) was used.
Gliosarcoma Brain Tumor Model.
Solitary intracerebral 9L tumors were established as described (23, 30). Briefly, 5 × 104 9L gliosarcoma cells in 2 μl of DMEM were stereotactically implanted into the right frontal lobe of adult male Fischer 344 rats. At 6 days after tumor implantation, osmotic pumps (DURECT; Alzet, Palo Alto, CA) were implanted with brain-infusion catheters inserted into the center of the tumor. Convection-enhanced delivery (36) was established by using a constant flow of 5 μl/hr for 3 days or 2.5 μl/hr for 6 days. Compounds infused into the tumor include PBS, 1.5 mg/ml T2, 2.0 mg/ml VEGF aptamer, 10.0 mg/ml integrin antagonist, or the triple combination. After treatment, frozen sections were stained with DAPI (nuclei) and anti Collagen IV, or anti-Ki67 (Novoste, Springfield, VA), followed by fluorescently labeled secondary antibodies (Invitrogen, Carlsbad, CA). Two experimental setups were used for the survival studies. In one, 8.0 μl/hr (Alzet) of solution was delivered for 24 h at 6 days and then again at 13 days after tumor implantation. In the other, a constant 2.5 μl/hr pump of triple combination or PBS was infused from days 6 to 12 after tumor implantation. For each survival study, the dates of mortality or severe morbidity (euthanasia) were recorded, and the results of two separate experiments were combined.
Supplementary Material
Acknowledgments
We thank members of the M.F. laboratory for helpful discussions and critical comments, Merck (Darmstadt, Germany) for providing us with the integrin antagonists, and Angiosyn (La Jolla, CA) for T2. This work was supported by National Eye Institute Grants EY11254 and EY14174, the Fonseca/Mericos Fund, and the V. Kann Rasmussen and MacTel Foundations (M.F.). F.B. is supported by the Skaggs Scholars in Clinical Science program.
Abbreviations
- OIR
oxygen-induced retinopathy
- Pn
postnatal day n
- T2
T2-TrpRS.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0607542104/DC1.
References
- 1.Carmeliet P. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Adamis AP, Aiello LP, D'Amato RA. Angiogenesis. 1999;3:9–14. doi: 10.1023/a:1009071601454. [DOI] [PubMed] [Google Scholar]
- 4.Das A, McGuire PG. Prog Retin Eye Res. 2003;22:721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Dorrell MI, Uj H, Aguilar E, Friedlander M. Surv Ophthalmol. 2007 doi: 10.1016/j.survophthal.2006.10.017. in press. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Kerbel RS. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld PJ, Heier JS, Hantsbarger G, Shams N. Ophthalmology. 2006;113:632. doi: 10.1016/j.ophtha.2006.01.027. e1. [DOI] [PubMed] [Google Scholar]
- 8.McCarty MF, Liu W, Fan F, Parikh A, Reimuth N, Stoeltzing O, Ellis LM. Trends Mol Med. 2003;9:53–58. doi: 10.1016/s1471-4914(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y. Semin Cancer Biol. 2004;14:139–145. doi: 10.1016/j.semcancer.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, et al. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 11.Stetler-Stevenson WG, Yu AE. Semin Cancer Biol. 2001;11:143–152. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 12.Ingber DE. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 13.Stupack DG, Cheresh DA. Curr Top Dev Biol. 2004;64:207–238. doi: 10.1016/S0070-2153(04)64009-9. [DOI] [PubMed] [Google Scholar]
- 14.Eyetech Study Group. Retina. 2002;22:143–152. doi: 10.1097/00006982-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 16.Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Proc Natl Acad Sci USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otani A, Slike BM, Dorrell MI, Hood J, Kinder K, Ewalt KL, Cheresh D, Schimmel P, Friedlander M. Proc Natl Acad Sci USA. 2002;99:178–183. doi: 10.1073/pnas.012601899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzima E, Reader JS, Irani-Tehrani M, Ewalt KL, Schwartz MA, Schimmel P. J Biol Chem. 2005;280:2405–2408. doi: 10.1074/jbc.C400431200. [DOI] [PubMed] [Google Scholar]
- 19.Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 20.Mulcahy MF, Benson AB., III Expert Opin Biol Ther. 2005;5:997–1005. doi: 10.1517/14712598.5.7.997. [DOI] [PubMed] [Google Scholar]
- 21.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 22.Madan A, Penn JS. Front Biosci. 2003;8:d1030–d1043. doi: 10.2741/1056. [DOI] [PubMed] [Google Scholar]
- 23.Barnett FH, Scharer-Schuksz M, Wood M, Yu X, Wagner TE, Friedlander M. Gene Ther. 2004;11:1283–1289. doi: 10.1038/sj.gt.3302287. [DOI] [PubMed] [Google Scholar]
- 24.Stromblad S, Fotedar A, Brickner H, Theesfeld C, Aguilar de Diaz E, Friedlander M, Cheresh DA. J Biol Chem. 2002;277:13371–13374. doi: 10.1074/jbc.C200044200. [DOI] [PubMed] [Google Scholar]
- 25.Zhong H, Bowen JP. Curr Med Chem. 2006;13:849–862. doi: 10.2174/092986706776361085. [DOI] [PubMed] [Google Scholar]
- 26.van Wijngaarden P, Coster DJ, Williams KA. J Am Med Assoc. 2005;293:1509–1513. doi: 10.1001/jama.293.12.1509. [DOI] [PubMed] [Google Scholar]
- 27.Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. J Clin Invest. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu K, Asai T, Oku N. Expert Opin Ther Targets. 2005;9:63–76. doi: 10.1517/14728222.9.1.63. [DOI] [PubMed] [Google Scholar]
- 29.Jain RK. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 30.Rainov NG, Ikeda K, Qureshi NH, Grover S, Herrlinger U, Pechan P, Chiocca EA, Breakefield XO, Barnett FH. Hum Gene Ther. 1999;10:311–318. doi: 10.1089/10430349950019093. [DOI] [PubMed] [Google Scholar]
- 31.Maulik N. Mol Cell Biochem. 2004;264:13–23. doi: 10.1023/b:mcbi.0000044370.20328.36. [DOI] [PubMed] [Google Scholar]
- 32.Zachary I. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- 33.Bridonneau P, Bunch S, Tengler R, Hill K, Carter J, Pieken W, Tinnermeier D, Lehrman R, Drolet DW. J Chromatogr B. 1999;726:237–247. [PubMed] [Google Scholar]
- 34.Dorrell MI, Aguilar E, Friedlander M. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- 35.Banin E, Dorrell MI, Aguilar E, Ritter MR, Aderman CM, Smith AC, Friedlander J, Friedlander M. Invest Ophthalmol Vis Sci. 2006;47:2125–2134. doi: 10.1167/iovs.05-1096. [DOI] [PubMed] [Google Scholar]
- 36.Saito R, Bringas JR, Panner A, Tamas M, Pieper RO, Berger MS, Bankiewicz KS. Cancer Res. 2004;64:6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.