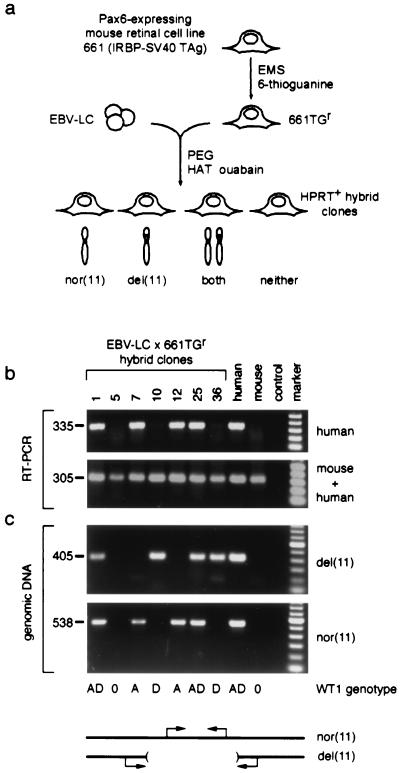
Figure 3.
Human PAX6 is not expressed from the deleted chromosome 11 in somatic cell hybrids. (a) Strategy used to make hybrid cell lines. EBV-lymphoblastoid cells from aniridia case 2 (EBV-LC) were fused to 661TGr, an HPRT-deficient mouse retinoblastoma cell line. Hybrid clones (n = 37) were selected in HAT + ouabain and classified on the basis of human chromosome 11 content. In the absence of selective pressure for human autosomes, both homologs were free to segregate. (b) RNA analysis of representative hybrids. Human PAX6 and mouse Pax6 expression is demonstrated by parallel RT-PCRs, using conditions that amplify human transcripts only or transcripts from both species (see Materials and Methods). Hybrid clones 1, 7, 12, and 25 retain the nor(11) and express PAX6, whereas clones 10 and 36 retain only the del(11) and do not express PAX6. Similar results were obtained for all 37 clones. Reactions performed on RNA from Ad12 (human) and 661TGr (mouse) retinal cells, and a no-RT control, are shown for comparison. The size of each PCR product is indicated in base pairs. (c) DNA analysis of representative hybrids. The chromosome 11 content is demonstrated by diagnostic genomic PCRs. An amplicon within the deletion identifies nor(11), and an amplicon spanning the breakpoint junction identifies del(11). PCRs performed on genomic DNA from case 2 (human) and 661TGr (mouse) cells, and a no-template control reaction, are shown for comparison. WT1 genotype data for the hybrid cell lines are indicated below the panels; the D allele is linked to the deletion. marker, 100-bp ladder (Roche); nor(11), normal; del(11), deleted.