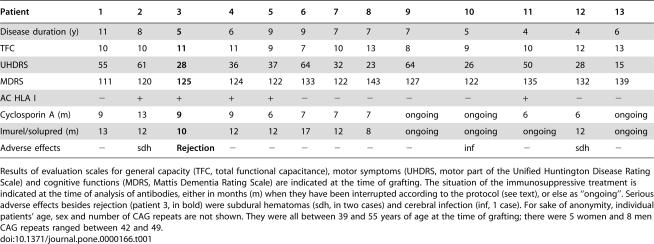
Table 1. Clinical characteristics of the 13 grafted patients tested for the presence of antibodies against foetal donors' HLA I antigens.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Disease duration (y) | 11 | 8 | 5 | 6 | 9 | 9 | 7 | 7 | 7 | 5 | 4 | 4 | 6 |
| TFC | 10 | 10 | 11 | 11 | 9 | 7 | 10 | 13 | 8 | 9 | 10 | 12 | 13 |
| UHDRS | 55 | 61 | 28 | 36 | 37 | 64 | 32 | 23 | 64 | 26 | 50 | 28 | 15 |
| MDRS | 111 | 120 | 125 | 124 | 122 | 133 | 122 | 143 | 127 | 122 | 135 | 132 | 139 |
| AC HLA I | − | + | + | + | + | − | − | − | − | − | + | − | − |
| Cyclosporin A (m) | 9 | 13 | 9 | 9 | 6 | 7 | 7 | 7 | ongoing | ongoing | 6 | 6 | ongoing |
| Imurel/solupred (m) | 13 | 12 | 10 | 12 | 12 | 17 | 12 | 8 | ongoing | ongoing | ongoing | 12 | ongoing |
| Adverse effects | − | sdh | Rejection | − | − | − | − | − | − | inf | − | sdh | − |
Results of evaluation scales for general capacity (TFC, total functional capacitance), motor symptoms (UHDRS, motor part of the Unified Huntington Disease Rating Scale) and cognitive functions (MDRS, Mattis Dementia Rating Scale) are indicated at the time of grafting. The situation of the immunosuppressive treatment is indicated at the time of analysis of antibodies, either in months (m) when they have been interrupted according to the protocol (see text), or else as “ongoing”. Serious adverse effects besides rejection (patient 3, in bold) were subdural hematomas (sdh, in two cases) and cerebral infection (inf, 1 case). For sake of anonymity, individual patients' age, sex and number of CAG repeats are not shown. They were all between 39 and 55 years of age at the time of grafting; there were 5 women and 8 men; CAG repeats ranged between 42 and 49.