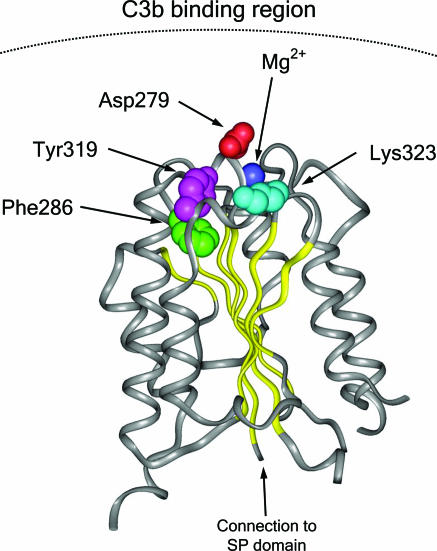
Fig. 2.
Structural implications for the HUS-associated BF mutations. Diagram of the von Willebrand type A domain of fB. Insight II (BYOSYM software package; Molecular Simulations, San Diego, CA) was used to draw structure by using PDB files 1Q0P and 1RS0_A. The positions of the residues Phe-286 and Lys-323 that are mutated in the HUS patients are indicated. The position of the Mg2+ ion and that of the Asp-279 and Tyr-319 are also indicated. Notice the edge-to-face stacking of Phe-286 and Tyr-319 residues. Numbering of residues is referred to with the initial methionine as “1” and therefore includes the sequence of the N-terminal signal peptide. Residue Asp-279 was described as Asp-254 by Hourcade et al. (32).