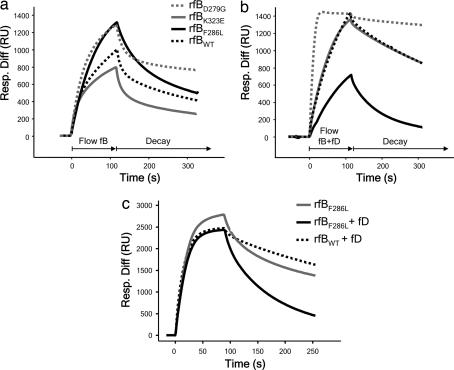
Fig. 4.
SPR analysis of recombinant fB proteins. (a) Formation of C3bB complexes. fBWT and fBK323E formed similar levels of the proenzyme, whereas fBF286L formed C3bB complexes much more rapidly and to a much higher level, similar to those formed by the fBD279G mutant described by Hourcade et al. (20). (b) Formation of C3bBb. At 10 μg/ml, fBWT and fBK323E formed similar levels of complexes with C3b. fBF286L formed lower levels of complexes with C3b in the presence than in the absence of fD as a consequence of accelerated rate of spontaneous decay of the cleaved fB. (c) Formation of C3bBb. At 37 μg/ml, fBF286L formed high levels of proenzyme, but when fD was included in the incubation, levels of activated enzyme formed by either recombinant fBWT or fBF286L were comparable. Resp. Diff, response difference; RU, resonance units.