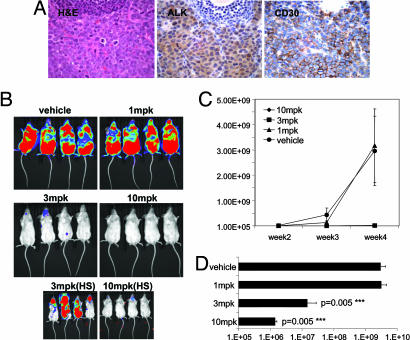
Fig. 5.
Effects of orally administered TAE684 on disease progression in an in vivo Karpas-299 lymphoma model. (A) Histopathology of an excised enlarged lymph node from a Fox Chase SCIDBeige mouse 4 weeks after an i.v. injection of one million Karpas-299 cells. Representative sections stained with H&E and against CD246 (ALK) or CD30 antigens are shown (magnification ×60). Images demonstrate strong infiltration of anaplastic, CD30- and ALK-positive Karpas-299 into the lymph node architecture. (B) Dose–response of the Karpas-299 lymphomas to 1, 3, and 10 mg/kg TAE684 or vehicle solution administered once daily. Dosing was initiated 3 days after mice received an i.v. injection of luciferase-expressing Karpas-299 cells. Representative low- and high-sensitivity bioluminescence images after 4 weeks of dosing are shown (n = 8 mice per group). (C) Effects of TAE684 or vehicle treatment on disease progression, estimated by weekly increases in bioluminescence signal with the Xenogen Imaging System (±SD). (D) Bioluminescence signal readout ± SD for mice shown in B after 4 weeks of treatment with either TAE684 or vehicle solution.