Figure 3.
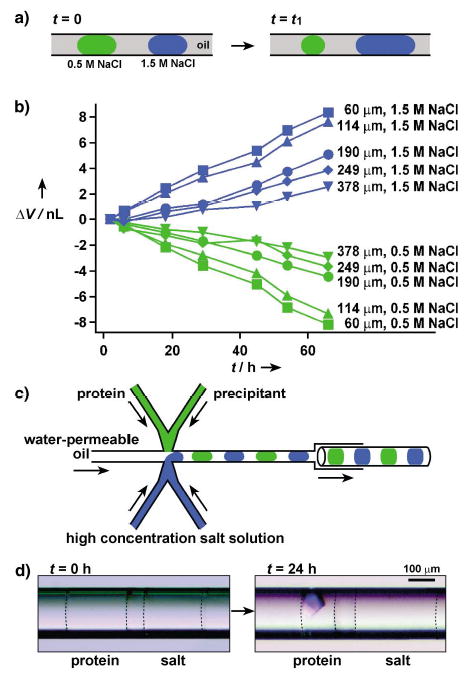
Protein crystallization by vapor diffusion in capillaries: a) A schematic illustration of the principle of vapor diffusion in a capillary. Water diffused through oil from droplets that contained a low concentration of salt to droplets that contained a high concentration of salt. b) A plot of change in the volume of droplets that were initially 25 nL (ΔV) as a function of time (t). Legends on the right side of each curve show the distance between the droplets. The concentrations of NaCl in the droplets are also indicated. c) A schematic illustration of the PDMS/glass composite device for setting up pairs of droplet for protein crystallization under vapor-diffusion conditions in capillaries. d) Two microphotographs that show a pair of droplets immediately after (left) and 24 h after (right) the setup. The droplet of the protein solution yielded a crystal after it had lost about 50% of its water content. Dashed lines were added to show the interface between the aqueous droplets and oil.
