Figure 3. Acetylation of lysine 120 catalyzed by hMOF and TIP60 increases in response to genotoxic stress.
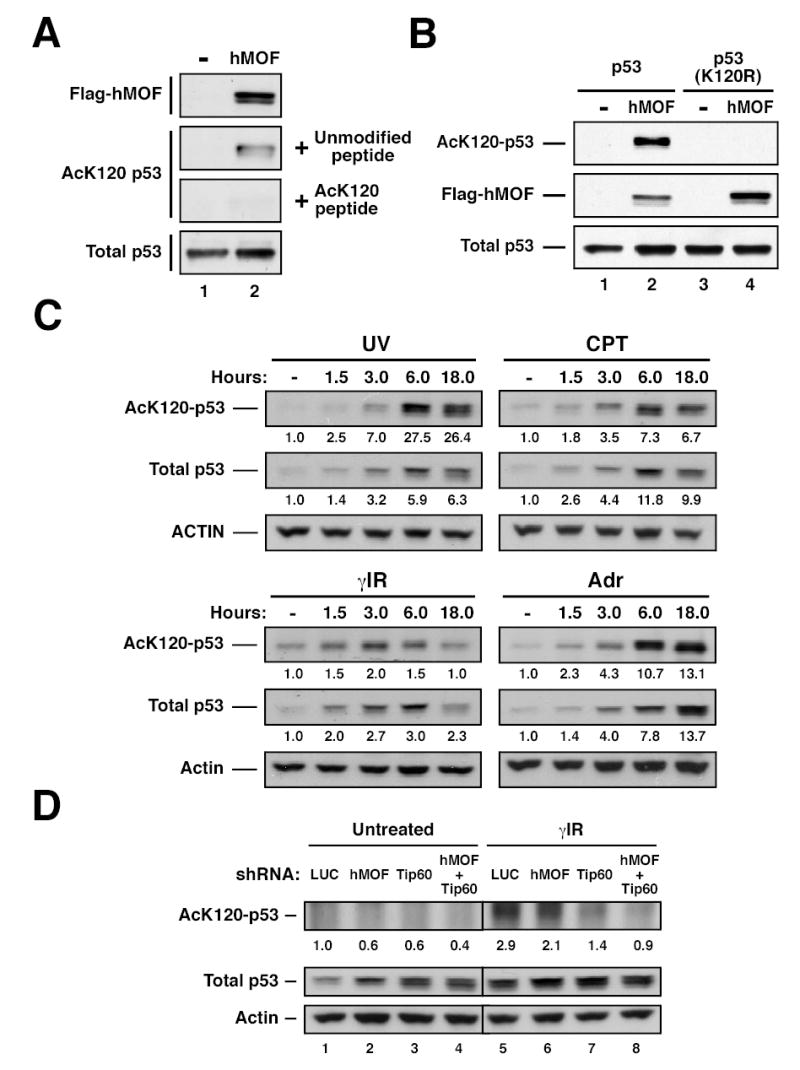
(A) H1299 cells were transfected with p53 in the presence or absence of hMOF. After transfection, cells were treated with 10mM sodium butyrate for 2–4 hours. Following treatment, the cell lysate was divided into two aliquots. One aliquot was subjected to immunoprecipitation (IP) with AcK120 antibody pre-blocked with 250μM modified peptide. The other aliquot was subjected to IP with AcK120 antibody pre-blocked with 25μM of unmodified peptide. Precipitates were then western blotted with a p53 antibody to quantify lysine-120 acetylation. Western blots were performed on the input lysates to ensure that equal amounts of p53 were used for each IP. (B) H1299 cells were transfected with wild-type p53 in the presence or absence of hMOF. In parallel, H1299 cells were co-transfected with p53-(K120R) and either vector control or hMOF. After transfection, cells were treated with 10mM sodium butyrate for 2–4 hours. Following treatment, cells were lysed and subjected to IP with an AcK120 antibody. Precipitates were western blotted with a p53 antibody to quantify lysine-120 acetylation. Input lysates were also subjected to western blot as described above. (C) U2OS cells were treated with 25 J/m2 UV, 5μM camptothecin (CPT), 10 Gy γ-irradiation (γIR), or 0.5 μg/ml adriamycin (Adr) and then harvested at the indicated times. Ninety minutes prior to each time point, cells were treated with 10mM Sodium Butyrate. K120 acetylation was assessed as described above. (D) U2OS cells were infected with recombinant lentiviruses that express shRNA molecules targeted to the corresponding genes. Seventy-two hours following infection, cells from each shRNA condition were treated with 10 Gy γ–Irradiation or left untreated. Two hours after irradiation, cells were treated with 10 mM Sodium Butyrate for 1 hour. Cells were then harvested and subjected to immunoprecipitation and subsequent western blot as described above.
