Abstract
Mate choice can lead to the evolution of sexual ornamentation. This idea rests on the assumption that individuals with more elaborate ornaments than competitors have higher reproductive success due to gaining greater control over mating decisions and resources provided by partners. Nevertheless, how the resources and quality of sexual partners that individuals gain access to are influenced by the ornamentation of rival individuals remains unclear. By experimentally concealing and subsequently revealing female ornaments to males, we confirm in the fowl, Gallus gallus, that female ornamentation influences male mating decisions. We further show, by manipulating the relative ornament size of females, that when females had larger ornaments than competitors they were more often preferred by males and obtained more sperm, especially from higher quality males, as measured by social status. Males may benefit by investing more sperm in females with larger ornaments as they were in better condition and produced heavier eggs. Female ornament size also decreased during incubation, providing a cue for males to avoid sexually unreceptive females. This study reveals how inter-sexual selection can lead to the evolution of female ornaments and highlights how the reproductive benefits gained from mate choice and bearing ornaments can be dependent upon social context.
Keywords: sexual selection, mate choice, female ornamentation, sperm allocation, incubation
1. Introduction
The disparity in the amount males and females invest per offspring is thought to account for why females typically display greater discrimination when choosing sexual partners than males (Trivers 1972), a topic which has attracted great interest over many years (Darwin 1871; Fisher 1958; Andersson 1994). More recently, it has been recognized that males may also exert choice over females when search costs for females are low, where females vary in reproductive quality and when males suffer mating and parental costs (Burley 1977; Parker 1983; Johnstone et al. 1996; Kokko & Monaghan 2001). Studies on male mate choice have found preferences for traits that reflect female fecundity such as body size and body mass (Verrell 1985; Bonduriansky 2001; Byrne & Rice 2006). Under such circumstances, female traits that determine fecundity may evolve via two distinct selection mechanisms: selection for increased fecundity and/or selection for attracting males (inter-sexual selection). Since both selection pressures exert positive directional selection on female traits, it is difficult to establish how important male mate choice is in shaping the evolution of female reproductive traits. The imprint of male mate choice on the evolution of female traits is much more easily determined when examining female ornamental traits, which exist in numerous species (Jones & Hunter 1993; Amundsen 2000b; Amundsen & Forsgren 2001; Lebas et al. 2003). Ornaments are costly to maintain and detract from female investment in fecundity (Chenoweth et al. 2006) and therefore ornamental traits are not likely to be under positive directional fecundity selection.
Theoretically, female ornaments are expected to evolve via male mate choice when their influence on male mating decisions and male-controlled resources raises their reproductive success beyond that obtainable from directly investing in offspring (Fitzpatrick et al. 1995). Empirical work across a range of taxa has demonstrated male preferences for female ornaments (Jones & Hunter 1993; Amundsen 2000b; Amundsen & Forsgren 2001; Domb & Pagel 2001). However, since it is often difficult to measure male mating preferences directly, surrogate measures of choice are used, such as time associated with females or copulation display rate, which may not necessarily reflect a male's decision to invest in a female (Amundsen 2000b). Ornaments are also often condition dependent and underpinned by traits such as body mass and size (Johnsen et al. 1996; Amundsen et al. 1997; Kotiaho 2000); therefore, experimental manipulations are important to identify preferences for ornaments and not correlated traits. Furthermore, central to the theory of the evolution of ornamentation via mate choice is the idea that individuals with relatively large ornaments gain a fitness advantage over individuals with smaller ornaments (Darwin 1871; Andersson 1994). Variation in social dynamics can result in individuals over their lifetime being associated with different competitors that may vary in their ornament expression. Under such circumstances, males may either assess female ornament size relative to those of other females or discriminate between females on their absolute ornament size. Which strategy males adopt is likely to depend on the costs associated with copulations relative to the rate they encounter females with different sized ornaments.
The aim of this study was to experimentally investigate using the model study system of the fowl Gallus gallus: (i) whether males base their mating decisions on female ornamentation, (ii) the influence of rival females' ornamentation on the probability of a female being chosen and the number of sperm she receives, and (iii) the reproductive benefits males gain from discriminating between females and how these change with variation in the ornamentation of available females.
Fowl have a highly flexible mating system that ranges from monogamy to, more commonly, intense promiscuity. Captive and wild populations of fowl live in highly social groups, which frequently change in size and composition from male–female pairs (Collias & Collias 1967, 1996; Collias & Saichuae 1967) up to flocks of 12 males and 16 females (Collias & Collias 1996) with, typically, groups of 1–2 males with between two and five females being observed (Johnson 1963; McBride et al. 1969; Ali & Ripley 1981; Collias & Collias 1996; Nishida et al. 2000). Therefore, males and females frequently experience situations where more than one copulation partner is simultaneously available. Males form dominance hierarchies and social status facilitates access to females, determining copulation success, and dominant males have higher fitness (Collias & Collias 1996). Males and females have fleshy head ornaments called combs and males with larger combs are preferred by females (Zuk et al. 1990). We have previously shown that males also display mate choice: the probability that males choose to copulate and the number of sperm males transfer during insemination is positively associated with female comb size (Pizzari et al. 2003; Cornwallis & Birkhead 2006). This may be beneficial for males as female comb size is correlated to egg mass (Pizzari et al. 2003). Females can lay several clutches of eggs in a breeding season and thus cycle through fertile and non-fertile periods (Madge & McGowan 2002). The complexity of the fowls' social and mating system results in males experiencing different social conditions and being exposed to females of different reproductive value, and it is predicted that males should be behaviourally and physiologically flexible to adjust to such variation in reproductive opportunities.
2. Material and methods
(a) Study population
We studied a free-ranging population of fowl that are behaviourally and morphologically very similar to red jungle fowl (Schütz & Jensen 2001) at Tovetorp Zoological Field Station, University of Stöckholm during May–August 2003. During the study, the population consisted of 35 males and 45 females and the age of males and females ranged from 1 to 4 years. Females were randomly assigned to groups of four and kept in aviaries (6×8 m) separated from pairs of males in adjoining aviaries (6×6 m) by wire netting. The dominance hierarchies of males were assessed through pairwise interactions. Female comb size was measured every two weeks by taking a digital image of the right and left side of the head against a standardized background with a measurement scale (400 mm2 square), under standardized lighting conditions. Comb size was calculated using Adobe Photoshop and the area of the comb in each image was calibrated against the measurement scale (Pizzari et al. 2003). An average of the comb size calculated from the right- and left-side images was used as the measure of a female's comb size on that day. Body mass was also measured every two weeks to the nearest 10 g, and body size was measured at the start and end of the breeding season using principal component one (PC1) of a principal components analysis of tarsus, wing and head measurements. PC1 explained 90.7% of the variation. For body mass and comb size, the measurements taken nearest to the time when females were presented to males were used in the analysis.
(b) Manipulation of the visibility of female ornaments
Females (n=18) were assigned to pairs (n=9) and the difference in the comb sizes of females was maximized (mean difference±s.e., 257±12 mm2). Females were not used if they showed signs of incubating eggs (see §2d). Females were ranked large and small according to their comb size. This experiment was conducted in two parts and took place between 16.00 and 20.30 h local time, the peak time of sexual activity (Parker et al. 1940). In the first part, each female was fitted with a cotton hood that concealed her comb. Males were kept in pairs, consisting of one dominant and one subordinate individual. Thirty minutes prior to presenting females, a male was chosen at random and removed from the aviary. Thirty minutes after being separated, the male that was left in the aviary was presented with a pair of females, fitted with hoods, first in a standing posture facing the male for 1 min to allow him to examine the females and then in a soliciting position with the tail facing the male. Each female was randomly assigned to one person who gently held the female throughout the trial allowing her posture to be carefully controlled. Females were fully habituated to being held and would readily feed from the hand. Females were placed 1 m apart from each other and were presented to the male when he was one metre away from the females. Males were allowed to mount females, but were prevented from copulating to stop males ejaculating. The first female mounted was considered the males' choice. In the second part, the females' hoods were removed and the procedure was repeated. The male that had been exposed to females was then switched with the other male in the pair and he was exposed to exactly the same treatment as the first male. Each pair of females was presented to between two and four different pairs of males.
We analysed differences in the probability of females being chosen by males (0,1) when their combs were visible and when they were concealed using a generalized linear mixed model (GLMM) with a binomial error distribution. Treatment (combs concealed and combs visible) and male social status were entered as fixed factors, difference in the body size and mass (large-combed female–small-combed female) as covariates, and female identity, specified as the subject of repeated measurement, as a random factor. Since the probability that one female chosen is dependent upon the other female, only variation in choice for females with the largest combs was analysed. To determine if males preferred females with the largest combs more often than females with small combs, under the visible and concealed treatments, the back-transformed least-squares (LS) means for each treatment was tested against a hypothetical mean of 50% using a one-sample t-test.
(c) Manipulation of female relative ornament size
The relative comb size of focal females (n=12) was manipulated by matching them with a female that had a larger and a smaller comb. With each female traid an attempt was made to maximize the difference in comb size between the focal female and females with larger and smaller combs (differences in comb sizes (mean±s.e. mm2): focal versus larger-combed females=+114±7; focal versus smaller-combed females=−78±6). Mate choice and sperm investment were assessed by presenting female pairs to males. Males were isolated from the other male in their pair 30 min prior to the presentation of females. Each of the females was held by one person and presented to males in a standing position for 1 min, enabling males to examine the females, following which the females were switched to a soliciting position and males were allowed to copulate. The first female a male copulated with was considered his choice. After the first copulation, a small wire cage was placed over the preferred female to encourage the male to copulate with the second female. After 48 h, the time taken by males to replenish their sperm stores (Etches 1996), the trial was repeated. However, the order that males copulated with females was reversed by initially placing the wire cage over the female the male copulated with first during the previous trial. Therefore, a male copulated four times in total, twice with each female 48 h apart, once when it was his first copulation of the day and once when it was his second copulation. The person holding each female was chosen at random for the first presentation. However, once the male had copulated, the person holding that female subsequently held the females during the remaining three copulations of the trial to control for any handling effects. Natural ejaculates were collected from males by fitting females with plastic harnesses that covered their cloacae and ejaculate volume was measured (μl) after each copulation using a Gilson pipette (Pizzari et al. 2003). Ejaculates were stored in 5% formalin solution for counting, which was done using a standard protocol (Bakst & Cecil 1997; Pizzari et al. 2003). Focal females were exposed to between four and eight different pairs of males, and with half the pairs of males focal females had smaller combs and in the other half they had larger combs.
We analysed the probability of focal females being chosen (0,1) when they had relatively large and small combs using a GLMM with a binomial error distribution, relative comb rank (large and small), male social status as fixed factors, difference in body mass and size between the focal and the other female as covariates, and focal female identity, specified as the subject, as a random factor. We also analysed the number of sperm focal females received relative to the comb size of the female they were presented with (percentage of sperm allocated to focal female–percentage of sperm allocated to other female) using a GLMM as the residuals of the model were normally distributed (Kolmogorov–Smirnov, D=0.07, p>0.05). Relative comb rank, male social status, copulation order and day of trial (day 1=1, 48 h later=2) were entered as fixed factors, the difference in body mass and body size between the focal and the other females were entered as covariates, and focal female identity was specified as a random factor.
(d) Female ornament size as a signal of fecundity
The potential reproductive benefits males acquire from basing their reproductive decisions on the ornament size of females were investigated by measuring female body mass, egg mass, mass of yolk in eggs and stage of reproductive cycle (laying to incubation) in relation to female comb size. The body mass, egg mass and yolk mass of focal females were compared with the females in the triad. Eggs were collected throughout the breeding season and wet egg and yolk mass were measured to the nearest 0.01 g. Unfortunately, it was not possible to obtain dry mass as with previous work (Pizzari et al. 2003). A mean egg and yolk mass was calculated for each female for the breeding season, which was used in all analyses. Maternity of eggs was assigned by feeding each female a different coloured fat soluble lipid dye in a gelatin capsule every 7 days, which stains the yolk of each developing egg (Gilbert 1972; Ward 1996).
We analysed the differences in body mass, egg mass and yolk mass between focal females and females with relatively large and small combs using GLMM. In the analysis of body mass, differences in body mass, difference in body size between focal and pair females as covariates, and focal female identity, specified as the subject, as a random factor. Differences in egg mass were analysed by entering comb rank as a fixed factor, difference in body mass and body size between focal and pair females as covariates, and focal female identity, specified as the subject, as a random effect. Differences in the mass of yolks produced by focal and pair females were analysed in the same way as egg mass, but difference in egg mass was entered as a covariate.
To assess whether female comb size signals the stage of the reproductive cycle, the comb size of individual females was monitored before, during and after they started incubating eggs. Incubation was induced by replacing eggs laid each day with model wooden eggs. The reproductive cycle of females was classified into four stages. (i) Before incubation: posture normal and no ‘clucking’ call, a distinct low-pitched call uttered repetitively and is associated with incubating and brooding chicks (Ramsay 1953). (ii) Start of incubation: females occasionally utter ‘clucking’ call and frequently visit and rearrange nest material. (iii) Incubating: females sit on eggs and only leave nest in the morning to feed and drink while continually uttering ‘clucking’ call. (iv) After incubation: same as stage (i). Incubation was terminated by removing eggs from females. The change in female comb size over the different stages of incubation was analysed using a GLMM, with comb size as the response variable, stage of incubation (i–iv) as a fixed factor, body mass and size as covariates, and female identity, specified as the subject, as a random effect.
All analyses were performed in SAS v. 9.1. Restricted maximum-likelihood estimation was used in GLMMs and restricted pseudo-likelihood estimation was used in GLMMs (Wolfinger & O'Connell 1993). The significance of fixed effects was examined using Wald-type tests (type III for main effects and type I for interactions; Grafen & Hails 2002) The fixed effect with the highest p-value was sequentially dropped until only significant terms (p<0.05) remained in the model.
3. Results
(a) Manipulation of visibility of female ornaments
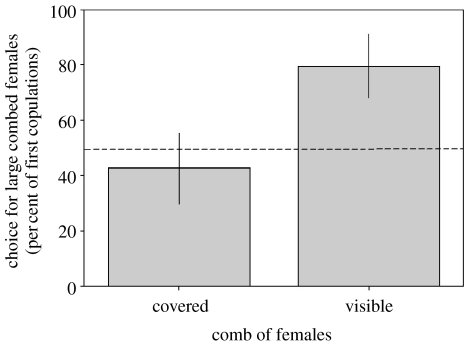
Male mating decisions were highly dependent on being able to observe the ornaments of females (table 1 and figure 1). When males were prevented from seeing the combs of females, the probability of choosing to copulate with the female with the largest comb was not significantly different from that expected by chance (probability of large-combed female receiving initial copulations in covered treatment (back-transformed LS mean±s.e.)=0.35±0.17. One-sample t-test against hypothetical probability of 0.5, t=0.89, d.f.=8, p=0.40; figure 1). However, when the combs of females were subsequently exposed, males displayed a significant preference for the female with the largest comb, indicating that the female comb plays an important role in male mating decisions (probability of large-combed female receiving initial copulations in visible treatment (back-transformed LS mean±s.e.)=0.87±0.09. One-sample t-test against hypothetical probability of 0.5, t=3.92, d.f.=8, p=0.004; figure 1).
Table 1.
The female comb as a target of male mate choice: statistical analysis of the effects of (a) visibility of the female comb on male mate choice for females with large combs, and female relative comb size on (b) male mate choice and (c) sperm allocation. (Parameter estimates are back-transformed and significant values are shown in boldface type.)
| analysis | term | parameter | effect ±s.e. | d.f. | F | p | |
|---|---|---|---|---|---|---|---|
| (a) visibility of female comb | treatment | hood | 0.35 | 0.17 | 1, 29 | 10.97 | 0.003 |
| no hood | 0.87 | 0.09 | |||||
| male status | — | — | 1, 28 | 0.92 | 0.34 | ||
| body mass difference | — | — | 1, 26 | 2.11 | 0.16 | ||
| body size difference | — | — | 1, 25 | 0.24 | 0.63 | ||
| treatment×male status | — | — | 1, 27 | 2.51 | 0.12 | ||
| (b) relative comb size: mate choice | comb rank | large | 0.72 | 0.08 | 1, 61 | 8.84 | 0.004 |
| small | 0.37 | 0.08 | |||||
| male status | — | — | 1, 60 | 0.73 | 0.39 | ||
| body mass difference | — | — | 1, 57 | 0.07 | 0.79 | ||
| body size difference | — | — | 1, 58 | 0.04 | 0.85 | ||
| comb rank×male status | — | — | 1, 59 | 0.08 | 0.78 | ||
| (c) relative comb size: Sperm allocation | comb rank | large | 0.21 | 0.09 | 1, 93 | 15.08 | 0.0002 |
| small | −0.14 | 0.09 | |||||
| male status | — | — | 1, 93 | 0.97 | 0.33 | ||
| copulation order | first | 0.36 | 0.08 | 1, 93 | 43.01 | <0.0001 | |
| second | −0.28 | 0.09 | |||||
| day of trial | — | — | 1, 91 | 0.45 | 0.50 | ||
| body mass difference | — | — | 1, 90 | 0.02 | 0.89 | ||
| body size difference | — | — | 1, 92 | 0.60 | 0.44 | ||
| comb rank×male status | large dom | 0.29 | 0.12 | 1, 93 | 4.92 | 0.03 | |
| large sub | 0.13 | 0.12 | |||||
| small dom | −0.28 | 0.11 | |||||
| small sub | 0.005 | 0.11 | |||||
| male status×copulation order | dom first | 0.23 | 0.11 | 1, 93 | 3.92 | 0.05 | |
| sub first | 0.48 | 0.11 | |||||
| dom second | −0.22 | 0.11 | |||||
| sub second | −0.35 | 0.12 | |||||
| day of trial×comb rank | — | — | 1, 88 | 0.00 | 0.98 | ||
| day of trial×status | — | — | 1, 89 | 0.02 | 0.90 | ||
| day of trial×comb rank×male status | — | — | 1, 87 | 0.88 | 0.35 |
Figure 1.
The probability of females with large and small combs being chosen by males when their combs were covered and when they were visible. When the combs of females were covered, males showed no preference for either large- or small-combed females. When the hoods were removed, males were significantly more likely to copulate with the female with the largest comb. Treatment (covered or visible), F1,29=10.97, p=0.003. Probability of large-combed female receiving initial copulations (back-transformed LS mean±s.e. tested versus 0.5): covered treatment, 0.35±0.17, t=0.89, d.f.=8, p=0.40; visible treatment, 0.87±0.09, t=3.92, d.f.=8, p=0.004.
(b) Manipulation of female relative ornament size
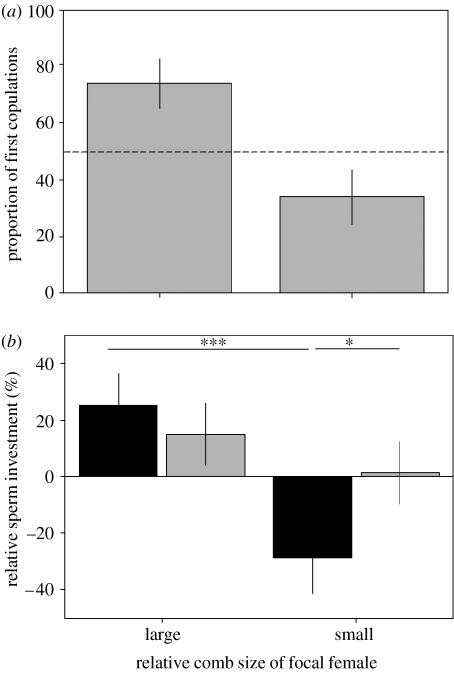
The relative size of a female's comb had a marked influence on the probability of her being preferred and the number of sperm she obtained from males of different social status. When females had larger combs than their pair female, the probability of them receiving initial copulations from both dominant and subordinate males was significantly higher than when their combs were smaller than the other female (table 1 and figure 2a). In addition, when females had relatively large combs, they received significantly more sperm from dominant males than subordinate males, in comparison with when they had small combs relative to their pair female (table 1 and figure 2b). Therefore, when females had relatively large combs they were more often chosen by dominant and subordinate males and received significantly more sperm, especially from dominant males, than when they had relatively smaller combs than rival females. The number of sperm females received from dominant and subordinate males when they had relatively large and relatively small combs was not different between days of the trial (first day versus 48 h later), indicating that when females had larger combs they did not gain more sperm simply because they were chosen a greater proportion of the time (table 1). This is consistent with previous evidence, which demonstrated that males allocated more sperm to females with large combs even when mate choice was removed (Cornwallis & Birkhead 2006). There were also strong effects of copulation order on the number of sperm males ejaculated and this was, to some extent, dependent upon male social status (table 1). Both dominant and subordinate males produced more sperm during initial copulations in comparison with second copulations, but this relationship was more pronounced in subordinate males as they allocated more sperm than dominant males to initial copulations (table 1).
Figure 2.
Effect of manipulating the relative comb size of focal females on (a) the probability of them being chosen and (b) the number of sperm they received from dominant (filled bars) and subordinate (grey bars) males. (a) Focal females were significantly more likely to receive the first copulation when presented with a female with a smaller comb than when presented with a female with a larger comb (comb rank, F1,61=8.84, p=0.004). (b) Focal females received more sperm when presented with females with smaller combs than when presented with females with larger combs (comb rank, F1,93=15.08, p=0.0002). Furthermore, the number of sperm focal females received was also dependent on the social status of the copulating male; when females had relatively small combs they received significantly less sperm from dominant males (comb rank×male social status, F1,93=4.92, p=0.03).
(c) Female ornament size as a signal of fecundity
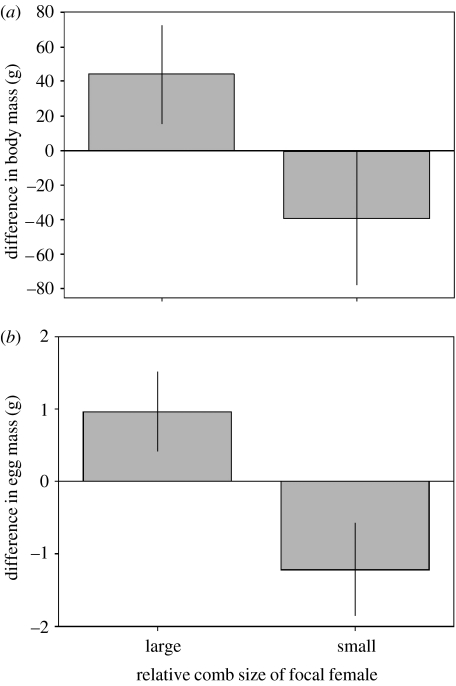
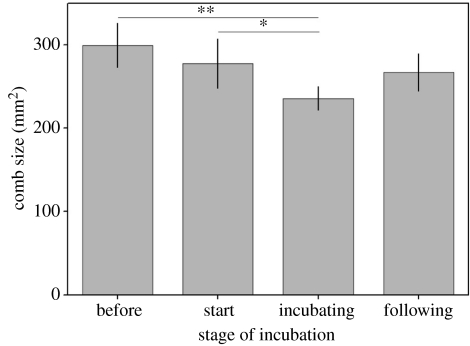
The body mass of focal females, after accounting for differences in body size, was significantly greater than that of females with smaller combs, but was less than females with larger combs (table 2 and figure 3a). Furthermore, after controlling for differences in body mass and size, focal females produced eggs that were significantly heavier than those laid by females with smaller combs, but lighter than the eggs produced by females with larger combs (table 2 and figure 3b). However, once differences in body mass and egg mass were taken into account, the amount of yolk in the eggs of focal females did not significantly differ from that of females with relatively large and small combs (table 2). Female comb size was also related to stage of incubation (table 2). When females began to incubate their comb size decreased and became significantly smaller than before incubation started (table 2 and figure 4). Once incubation was terminated the comb size of females started to increase (table 2 and figure 4).
Table 2.
Female comb size as a signal of fecundity: statistical analysis of the difference in (a) body mass, (b) egg mass and (c) yolk mass between females with relatively large and small combs and (d) the changes in female comb size across stages of incubation. (Significant values are shown in boldface type.)
| analysis | term | parameter | effect ±s.e. | d.f. | F | p | |
|---|---|---|---|---|---|---|---|
| (a) body mass | comb rank | large | 46.74 | 27.38 | 1, 16 | 8.06 | 0.006 |
| small | −34.90 | 26.55 | |||||
| body size difference | 6.82 | 1.68 | 1, 16 | 16.55 | 0.0009 | ||
| (b) egg mass | comb rank | large | 1.01 | 0.55 | 1, 17 | 13.96 | 0.002 |
| small | −1.25 | 0.53 | |||||
| body mass difference | 0.008 | 0.004 | 1, 15 | 4.33 | 0.055 | ||
| body size difference | — | — | 1, 15 | 3.05 | 0.10 | ||
| (c) yolk mass | comb rank | — | — | 1, 15 | 2.21 | 0.16 | |
| body mass difference | 0.002 | 0.001 | 1, 16 | 4.89 | 0.04 | ||
| body size difference | — | — | 1, 14 | 0.37 | 0.56 | ||
| egg mass difference | 0.22 | 0.07 | 1, 16 | 11.87 | 0.004 | ||
| (d) stage of incubation | stage of incubation | before | 295.28 | 20.39 | 3, 50 | 4.34 | 0.009 |
| start | 289.34 | 23.70 | |||||
| incubating | 251.34 | 20.36 | |||||
| after | 269.58 | 20.84 | |||||
| body mass | 0.48 | 0.09 | 1, 50 | 29.87 | <0.0001 | ||
| body size | — | — | 1, 50 | 0.01 | 0.91 | ||
Figure 3.
The difference in (a) body mass and (b) egg mass between focal females and females with relatively large and small combs. (a) Focal females, after controlling for differences in body size, were significantly heavier than females with smaller combs and significantly lighter than females with relatively larger combs (comb rank, F1,16=10.14, p=0.006). (b) The eggs produced by focal females were of significantly greater mass when compared with females with relatively smaller combs and significantly lower mass when compared with those from females with relatively larger combs (comb rank, F1,17=13.96, p=0.002).
Figure 4.
The change in female comb size over the different stages of incubation. The size of a female's comb significantly declined as incubation progressed until incubation was terminated, after which comb size increased again (stage of incubation, F3,50=4.34).
4. Discussion
Our results show that the comb of the female fowl is a target of male mate choice. The success of females in attracting and obtaining sperm from males was, however, highly dependent upon the comb size of competing females. Preferentially, investing in females that have the largest combs at any point in time appears adaptive for males as these females were in the better condition and laid heavier eggs. Furthermore, females with larger combs were more likely to be sexually receptive as female comb size decreased during incubation.
Central to the theory of the evolution of ornamentation is the assertion that individuals gain a fitness advantage by having larger ornaments than competitors (Amundsen 2000a). Despite this, there have been few studies that have examined experimentally how the reproductive benefits that individuals acquire change when the expression of their ornamentation varies in relation to competitors. Evidence from this study suggests that females possessing larger ornaments than competitors may be able to gain copulations prior to other females and to obtain more sperm, especially from dominant males, which is likely to hold certain reproductive benefits for females. Obtaining more sperm and obtaining sperm before other females may provide direct benefits for females by ensuring fertility (Sheldon 1994) and reducing costs associated with seeking copulations with males. Sexually active male fowl frequently copulate and it is likely that they always experience some degree of sperm depletion, i.e. they never have the same numbers of sperm as fully rested males and hence probably never have surplus sperm (Pizzari et al. 2003). Therefore, if females fail to gain initial copulations with males, they run a greater risk of infertility. In addition, by acquiring more sperm from dominant males, females may gain indirect benefits. Social dominance in the fowl is heritable (Craig et al. 1965) and dominant individuals have higher lifetime reproductive success than subordinate individuals (Collias & Collias 1996).
The discrepancy between the amount of sperm that dominant and subordinate males allocated to females may be explained by the effect of copulation order on male sperm investment (Cornwallis & Birkhead 2006). We have previously shown that subordinate males primarily allocate sperm to initial copulations, whereas dominant males are less influenced by copulation order and invest sperm according to the reproductive value of females (Cornwallis & Birkhead 2006). Therefore, over a series of copulations, dominant males allocate more sperm than subordinate males to females with large combs. Consistent with this idea, there was a significant interaction between social status and copulation order in this study (table 1).
Female comb size was an indicator of body condition and egg quality relative to other available females, which is in accordance with previous research (Pizzari et al. 2003). It may therefore be advantageous for males to use female comb size as a criterion in mating decisions. By copulating with females that are in better condition, males may reduce exposure to diseases (Sheldon 1993; Potti & Merino 1996) and invest in females that are more likely to overcome the energetic processes of egg laying, incubation and chick rearing, which in the fowl is solely done by the female (Linville et al. 1998). Males may also gain superior offspring by reproducing with females with large combs as they produce heavier eggs and, in a number of species, egg mass correlates with survival and fledging weight (Parsons 1970; Moss et al. 1981; Bolton 1991). Furthermore, by assessing females by their comb size, males may avoid females that are not fertile as comb size regresses as females start to incubate.
The reduction in comb size during incubation is most likely due to a reduction in food intake, which leads to a decrease in body condition, combined with changes in hormone levels (Etches 1996). As egg laying progresses, levels of prolactin rise until they cause a decline in luteinizing hormone, which results in ovarian collapse (Burke et al. 1981). Ovarian follicles are one of the main sources of steroid hormones including androgens and the expression of the comb is androgen dependent (Johnson 2000). Therefore, the decline in ovarian activity and steroid production is likely to lead to the regression of the comb. A reduction in comb size during incubation may allow females to (i) conserve energy resources (Etches 1996), (ii) reduce the chances of predation as the comb is highly visible and females nest on the ground, and (iii) decrease levels of male harassment which can be intense in the fowl (Pizzari & Birkhead 2000).
Fowl have a highly flexible social structure and the composition of groups often changes (McBride et al. 1969). Males frequently have the opportunity to mate with multiple females in both captive and wild populations (Collias & Collias 1967, 1996; Ali & Ripley 1981; Pizzari et al. 2002). Male investment in individual females was highly dependent on the other females present. This may be due to the way female quality is assessed, with males being able to distinguish between females only when they can visually compare one against the other. Alternatively, it may pay males to invest in the best reproductive opportunity currently available, even when males may encounter females of higher quality in the future, because the probability of gaining copulations with such females is uncertain (Reinhold et al. 2002). Males also continually replenish their sperm reserves, possibly enabling them to invest sperm in these opportunities if and when they arise. Male choice for females with the largest combs suggests that there is directional sexual selection for increased female comb size. However, the strength of sexual selection appears to be dependent upon the relative frequency of females with different ornament sizes. Although this species is highly social, providing males with the opportunity to simultaneously assess and discriminate between females with different ornament sizes, single females are observed with males (Collias & Collias 1967, 1996). Under such circumstances, it would appear that females with small ornaments may acquire similar amounts of sperm from males as females with large ornaments, and this may provide an explanation for how variation is maintained in a trait that is under directional selection (Andersson 1994). These results were gained under experimental conditions and it will now be important to quantify changes in social dynamics and assess how this generates variation in the relative ornament sizes of females that males are exposed to under natural conditions.
Acknowledgments
We thank Sven Jakobsson for support at Tovetorp Zoological Research Station, University of Stöckholm, Emily O'Connor, Kirsi Kupsala, Riikka Mikkonen and Hanne Løvlie for field assistance. This work was supported by a grant from the Natural Environmental Research Council.
References
- Ali S, Ripley S.D. Oxford University Press; Oxford, UK: 1981. Handbook of the birds of India and Pakistan together with those of Bangladesh, Nepal, Bhutan and Sri Lanka. [Google Scholar]
- Amundsen T. Female ornaments: genetically correlated or sexually selected? In: Espmark Y, Amundsen T, Rosenqvist G, editors. Animal signals: signalling and signal design in animal communication. Tapir Academic Press; Trondheim, Norway: 2000a. pp. 133–154. [Google Scholar]
- Amundsen T. Why are female birds ornamented? Trends. Ecol. Evol. 2000b;15:149–155. doi: 10.1016/s0169-5347(99)01800-5. doi:10.1016/S0169-5347(99)01800-5 [DOI] [PubMed] [Google Scholar]
- Amundsen T, Forsgren E. Male mate choice selects for female coloration in a fish. Proc. Natl Acad. Sci. USA. 2001;98:13 155–13 160. doi: 10.1073/pnas.211439298. doi:10.1073/pnas.211439298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundsen T, Forsgren E, Hansen L.T.T. On the function of female ornaments: male bluethroats prefer colourful females. Proc. R. Soc. B. 1997;264:1579–1586. doi:10.1098/rspb.1997.0220 [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Bakst M.R, Cecil H.C, editors. Techniques for semen evaluation, semen storage, and fertility determination. 4. Sperm motility and metabolism VII. In vitro sperm-egg interaction assay utilising inner perivitelline layer from laid chicken eggs. Poultry Science Asociation; Savoy, IL: 1997. [Google Scholar]
- Bolton M. Determinants of chick survival in the lesser black-backed gull—relative contributions of egg size and parental quality. J. Anim. Ecol. 1991;60:949–960. doi:10.2307/5424 [Google Scholar]
- Bonduriansky R. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. Camb. Philos. Soc. 2001;76:305–339. doi: 10.1017/s1464793101005693. doi:10.1017/S1464793101005693 [DOI] [PubMed] [Google Scholar]
- Burke W.H, Dennison P.H, Silsby J.L, El Halawani M.E. Serum prolactin levels of turkey hens in relation to reproductive function. Adv. Physiol. Sci. 1981;33:109–116. [Google Scholar]
- Burley N. Parental investment, mate choice, and mate quality. Proc. Natl Acad. Sci. USA. 1977;74:3476–3479. doi: 10.1073/pnas.74.8.3476. doi:10.1073/pnas.74.8.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P.G, Rice W.R. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc. R. Soc. B. 2006;273:917–922. doi: 10.1098/rspb.2005.3372. doi:10.1098/rspb.2005.3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth S.F, Doughty P, Kokko H. Can non-directional male mating preferences facilitate honest female ornamentation? Ecol. Lett. 2006;9:179–184. doi: 10.1111/j.1461-0248.2005.00867.x. doi:10.1111/j.1461-0248.2005.00867.x [DOI] [PubMed] [Google Scholar]
- Collias N.E, Collias E.C. A field study of the red jungle fowl in North-Central India. Condor. 1967;69:360–386. [Google Scholar]
- Collias N.E, Collias E.C. Social organization of a red junglefowl, Gallus gallus, population related to evolutionary theory. Anim. Behav. 1996;51:1337–1354. doi:10.1006/anbe.1996.0137 [Google Scholar]
- Collias N.E, Saichuae P. Ecology of the red jungle fowl in Thailand and Malaya with reference to the origin of domestication. Nat. Hist. Bull. Siam Soc. 1967:189–209. [Google Scholar]
- Cornwallis C.K, Birkhead T.R. Social status and availability of females determine patterns of sperm allocation in the fowl. Evolution. 2006;60:1486–1493. doi:10.1554/06-098.1 [PubMed] [Google Scholar]
- Craig J.V, Ortman L.L, Guhl A.M. Genetic selection for social dominance ability in chicks. Anim. Behav. 1965;13:114–131. doi:10.1016/0003-3472(65)90081-3 [Google Scholar]
- Darwin C. John Murray; London, UK: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Domb L.G, Pagel M. Sexual swellings advertise female quality in wild baboons. Nature. 2001;410:204–206. doi: 10.1038/35065597. doi:10.1038/35065597 [DOI] [PubMed] [Google Scholar]
- Etches R.J. CAB International; Oxford, UK: 1996. Reproduction in poultry. [Google Scholar]
- Fisher R.A. Dover; New York, NY: 1958. The genetical theory of natural selection. [Google Scholar]
- Fitzpatrick S, Berglund A, Rosenqvist G. Ornaments or offspring: costs to reproductive success restrict sexual selection processes. Biol. J. Linn. Soc. Lond. 1995;55:251–260. [Google Scholar]
- Gilbert A.B. The activity of the ovary in relation to egg production. In: Freeman B.M, Lake P.E, editors. Egg formation and production. British Poultry Science; Edinburgh, UK: 1972. pp. 3–21. [Google Scholar]
- Grafen A, Hails R. Oxford University Press; Oxford, UK: 2002. Modern statistics for the life sciences. [Google Scholar]
- Johnsen T.S, Hengeveld J.D, Blank J.L, Yasukawa K, Nolan V. Epaulet brightness and condition in female red-winged blackbirds. Auk. 1996;113:356–362. [Google Scholar]
- Johnson R.A. Habitat preference and behaviour of breeding jungle fowl in central western Thailand. The Wilson Bull. 1963;75:270–273. [Google Scholar]
- Johnson A.L. Reproduction in the female. In: Sturkie P.D, editor. Avian physiology. Academic Press; London, UK: 2000. pp. 569–596. [Google Scholar]
- Johnstone R.A, Reynolds J.D, Deutsch J.C. Mutual mate choice and sex differences in choosiness. Evolution. 1996;50:1382–1391. doi: 10.1111/j.1558-5646.1996.tb03912.x. doi:10.2307/2410876 [DOI] [PubMed] [Google Scholar]
- Jones I.L, Hunter F.M. Mutual sexual selection in a monogamous seabird. Nature. 1993;362:238–239. doi:10.1038/362238a0 [Google Scholar]
- Kokko H, Monaghan P. Predicting the direction of sexual selection. Ecol. Lett. 2001;4:159–165. doi:10.1046/j.1461-0248.2001.00212.x [Google Scholar]
- Kotiaho J.S. Testing the assumptions of conditional handicap theory: costs and condition dependence of a sexually selected trait. Behav. Ecol. Sociobiol. 2000;48:188–194. doi:10.1007/s002650000221 [Google Scholar]
- Lebas N.R, Hockham L.R, Ritchie M.G. Nonlinear and correlational sexual selection on ‘honest’ female ornamentation. Proc. R. Soc. B. 2003;270:2159–2165. doi: 10.1098/rspb.2003.2482. doi:10.1098/rspb.2003.2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linville S.U, Breitwisch R, Schilling A.J. Plumage brightness as an indicator of parental care in northern cardinals. Anim. Behav. 1998;55:119–127. doi: 10.1006/anbe.1997.0595. doi:10.1006/anbe.1997.0595 [DOI] [PubMed] [Google Scholar]
- Madge S, McGowan P. Helm; London, UK: 2002. Pheasants, partridges & grouse. [Google Scholar]
- McBride G, Parer I.P, Foenander F. The social organisation and behaviour of the feral domestic fowl. Anim. Behav. Monogr. 1969;2:125–181. [Google Scholar]
- Moss R, Watson A, Rothery P, Glennie W.W. Clutch size, egg size, hatch weight and laying date in relation to early mortality in red grouse, Lagopus lagopus scoticus, chicks. Ibis. 1981;123:450–462. [Google Scholar]
- Nishida T, et al. Morphological Identification and Ecology of the Red Jungle Fowl in Thailand, Loas and Vietnam. Anim. Sci. J. 2000;71:470–480. [Google Scholar]
- Parker G.A. Mate quality and mating decisions. In: Bateson P, editor. Mate choice. Cambridge University Press; New York, NY: 1983. pp. 141–166. [Google Scholar]
- Parker J.E, McKenzie F.F, Kempster H.L. Observations on the sexual behaviour of New Hampshire males. Poult. Sci. 1940;19:191–197. [Google Scholar]
- Parsons J. Relationship between egg size and post hatching chick mortality in the herring gull (Larus argentatus) Nature. 1970;228:1221–1222. doi: 10.1038/2281221a0. doi:10.1038/2281221a0 [DOI] [PubMed] [Google Scholar]
- Pizzari T, Birkhead T.R. Female fowl eject sperm of subdominant males. Nature. 2000;405:787–789. doi: 10.1038/35015558. doi:10.1038/35015558 [DOI] [PubMed] [Google Scholar]
- Pizzari T, Froman D.P, Birkhead T.R. Pre- and post-insemination episodes of sexual selection in the fowl. Heredity. 2002;89:112–116. doi: 10.1038/sj.hdy.6800014. doi:10.1038/sj.hdy.6800014 [DOI] [PubMed] [Google Scholar]
- Pizzari T, Cornwallis C.K, Lovlie H, Jakobsson S, Birkhead T.R. Sophisticated sperm allocation in male fowl. Nature. 2003;426:70–74. doi: 10.1038/nature02004. doi:10.1038/nature02004 [DOI] [PubMed] [Google Scholar]
- Potti J, Merino S. Decreased levels of blood trypanosome infection correlate with female expression of a male secondary sexual trait: implications for sexual selection. Proc. R. Soc. B. 1996;263:1199–1204. [Google Scholar]
- Ramsay A.O. Variations in the development of broodiness in fowl. Behaviour. 1953;5:51–57. [Google Scholar]
- Reinhold K, Kurtz J, Engqvist L. Cryptic male choice: sperm allocation strategies when female quality varies. J. Evol. Biol. 2002;15:201–209. doi:10.1046/j.1420-9101.2002.00390.x [Google Scholar]
- Schütz K.E, Jensen P. Effects of resource allocation on behavioural strategies: a comparison of red junglefowl (Gallus gallus) and two domesticated breeds of poultry. Ethology. 2001;107:753–765. doi:10.1046/j.1439-0310.2001.00703.x [Google Scholar]
- Sheldon B.C. Sexually transmitted disease in birds: occurence and evolutionary significance. Phil. Trans. R. Soc. B. 1993;339:491–497. doi: 10.1098/rstb.1993.0044. [DOI] [PubMed] [Google Scholar]
- Sheldon B.C. Male phenotype, fertility and the pursuit of extra-pair copulations by female birds. Proc. R. Soc. B. 1994;257:25–30. [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine-Atherton; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Verrell P.A. Male mate choice for large, fecund females in the red-spotted newt, Notophthalmus viridescens, how is size assessed. Herpetologica. 1985;41:382–386. [Google Scholar]
- Ward S. Energy expenditure of female barn swallows, Hirundo rustica, egg formation. Physiol. Zool. 1996;69:930–951. [Google Scholar]
- Wolfinger R, O'Connell M. Generalised linear mixed models: a pseudo-likelihood approach. J. Stat. Comput. Simul. 1993;4:233–243. [Google Scholar]
- Zuk M, Johnson K, Thornhill R, Ligon J.D. Mechanisms of female choice in red jungle fowl. Evolution. 1990;44:477–485. doi: 10.1111/j.1558-5646.1990.tb05933.x. doi:10.2307/2409430 [DOI] [PubMed] [Google Scholar]