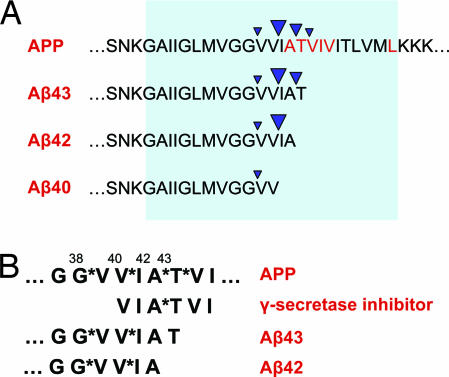
Fig. 5.
Longer forms of Aβ could act as competitive inhibitors of γ-secretase. (A) The diagram shows the APP amino acid sequence surrounding the sites of intramembranous cleavage by γ-secretase and the major Aβ peptides produced. The intramembranous portion of APP is indicated by the shaded region. Cleavage sites are indicated by inverted arrowheads, with the size of the arrowhead denoting the relative frequency of each cleavage site. Under normal circumstances, Aβ40 is the predominant species produced by γ-secretase cleavage, and Aβ peptides of 43, 42, and 38 residues in length are produced in lower amounts. FAD-linked mutations in PS and APP typically enhance the production of Aβ42 and Aβ43. APP residues at which FAD-linked mutations have been identified are highlighted in red. Interestingly, the longer forms of Aβ (Aβ42, Aβ43) retain the major cleavage site for generation of Aβ40. Although they may be capable of interacting with the enzyme active site, these longer forms of Aβ are unlikely to be efficient substrates for cleavage owing to the absence of distal residues, raising the possibility that they may occupy the active site nonproductively after their generation. Thus, Aβ42 and Aβ43 may act as γ-secretase inhibitors, and their increased production in familial and sporadic AD may result in inhibition of γ-secretase. (B) The amino acid sequence of APP surrounding the multiple intramembranous γ-secretase cleavage sites is diagrammed at top, with the cleavage sites designated by asterisks; the length of the corresponding Aβ peptide is indicated above the sequence. The structure of a typical substrate-based γ-secretase inhibitor and the C-terminal sequences of Aβ43 and Aβ42 are shown below. Substrate-based γ-secretase inhibitors are peptide analogs typically derived from the amino acid sequence surrounding the Aβ42 cleavage site. Residues immediately flanking the cleavage site (A*T) are often modified to incorporate hydroxyethyl isostere or difluoroketone moieties, which mimic the transition state intermediate in aspartic protease catalysis. The C-terminal sequences of Aβ43 and Aβ42 resemble substrate-based peptide inhibitors directed against the Aβ38–Aβ40 cleavage sites.