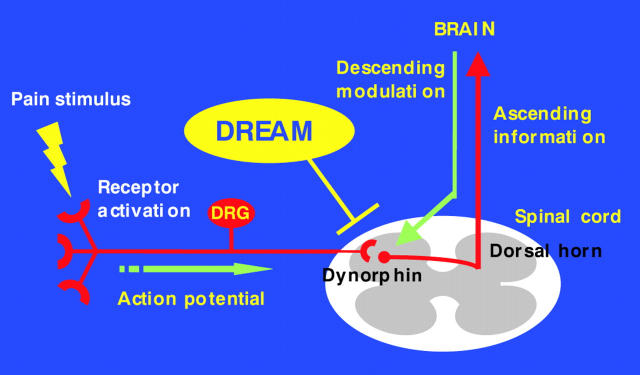
Figure 1.
A model for downstream regulatory element antagonistic modulator (DREAM) dependent modulation of pain transmission at the spinal level. Noxious stimulation of peripheral tissues activates peripheral sensory afferents and evokes the release of excitatory neurotransmitters from their central terminals onto the spinal dorsal horn. The nociceptive information is eventually relayed to higher pain processing centres within the brain, which, in turn, can provide descending modulation of nociceptive transmission at the spinal level. Generally speaking, spinal opioids such as dynorphin have inhibitory effects on nociceptive transmission. DREAM is expressed in the spinal cord and controls the expression of the prodynorphin gene. Loss of DREAM function—for example, by genetic deletion in our mouse model—results in elevated expression of prodynorphin and enhanced κ-opioid receptor activation under basal conditions. As a consequence, DREAM deficient mice exhibit a phenotype of "ongoing analgesia". DRG, dorsal root ganglion.