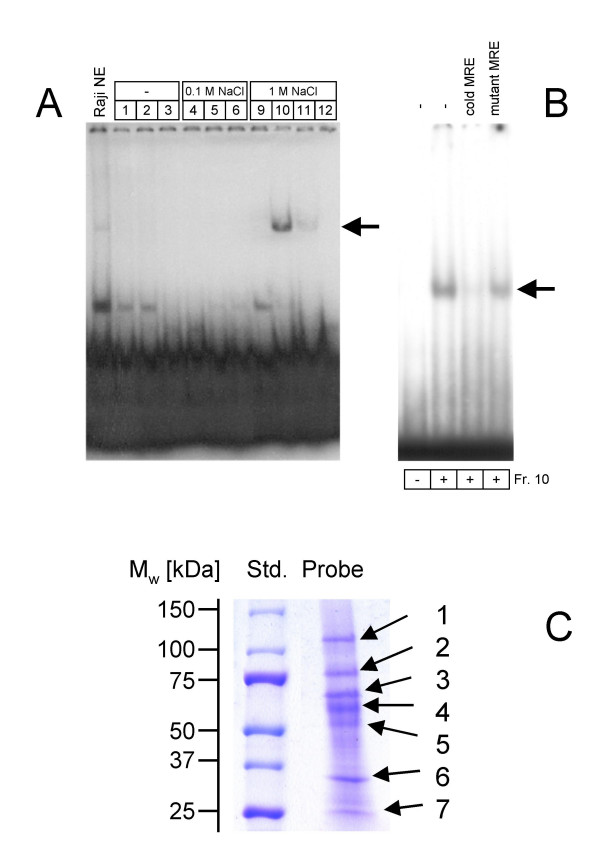
Figure 1.
(A) DNA affinity chromatography. Nuclear proteins (20 mg total protein) were prepared from Raji cells, and subjected to DNA affinity chromatography with the biotinylated MRE oligonucleotide (position -400 to -357 bp). Proteins specifically bound to MRE were eluted with different NaCl concentrations, and 10 μl-aliquots (800 μl total) were analyzed by EMSA. (B) Competition EMSA analysis. For competition analysis of MRE-binding, either unlabelled MRE oligonucleotide or MRE mutant oligonucleotide was added at a 100-fold molar excess to the binding reactions. (C) Separation of the proteins bound to MRE. The 1M NaCl eluate of affinity chromatography was precipitated with UPPA-Protein Concentrate Kit according to the manufacturer's protocol. The precipitate was dissolved in SDS-PAGE sample buffer and subjected to SDS-PAGE (Ready-Gel gradient gel 4–15% (Biorad, Munich, Germany). The proteins were stained by Coomassie R250 The proteins are numbered according their molecular weights.