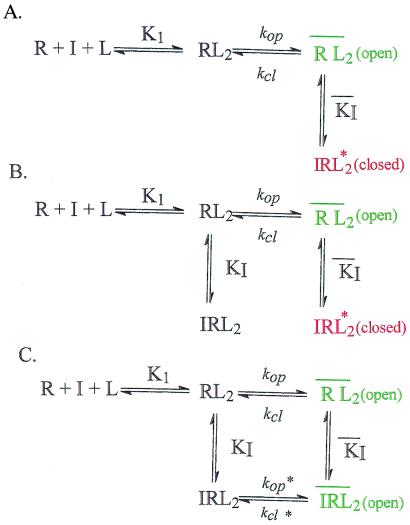
Figure 1.
Proposed mechanisms for the inhibition of AChR by MK-801 and cocaine. In each case, the upper line represents the minimum mechanism for the opening of the receptor-channel (10). Receptor R binds the neurotransmitter L (or another activating ligand, for instance carbamoylcholine). Katz and Thesleff (10) first suggested the binding of at least two ligand molecules to the receptor before the receptor-channel opens. R, RL, and RL2 represent the closed-channel conformations. RL2 represents the open-channel conformation of the receptor that allows inorganic cations to cross the cell membrane, thus initiating an electrical signal and intercellular communication. K1 is the observed dissociation constant for the activating ligand. kop and kcl are the rate constants for channel opening and closing, respectively; Φ−1 (=kop/kcl) is the channel-opening equilibrium constant (25, 43). The reactions shown occur in the microsecond-to-millisecond time region (13, 22–24, 44). For clarity, the desensitization reaction, which in the case of the AChR occurs in the 100- to 500-ms time region (44, 45), and the binding of the inhibitor I to the unliganded receptor form are not shown. The relatively slow transitions of receptor/inhibitor complexes to nonconductive forms (6, 9, 30) are also not shown. (A) Channel-blocking mechanism in which the inhibitor binds in the open channel and blocks it (14). (B) Extended channel-blocking mechanism. The inhibitor binds to the closed- and open-channel forms giving the nonconducting receptor forms IRL2 and IRL*2 (18). KI and KI are the observed inhibitor dissociation constants pertaining to the closed- and open-channel form, respectively. (C) Proposed cyclic inhibition mechanism involving a complex of the inhibitor with the open-channel conformation in which the open channel is not blocked by the inhibitor (i.e., it conducts ions). This minimum mechanism is based on chemical kinetic measurements and on predictions it makes regarding the properties of ligands that will inhibit the receptor and of those that will not inhibit the receptor but will prevent the binding of inhibitors. The principle of microscopic reversibility (37) requires that the ratio and KI/KI = Φ−1/ΦI0−1 where ΦI0−1 = k*op/k*cl. Therefore compounds that bind to a regulatory site with higher affinity for the closed-channel conformation than the open-channel form will shift the equilibrium toward the closed-channel form and inhibit the receptor. Compounds that bind to the open-channel conformation with equal or higher affinity than to the closed-channel form are expected to displace inhibitors from the regulatory sites without inhibiting receptor activity.