Abstract
Objective: To evaluate the prevalence and correlates of left ventricular thrombosis in patients with acute myocardial infarction, and whether the occurrence of early mitral regurgitation has a protective effect against the formation of left ventricular thrombus.
Design and setting: Multicentre clinical trial carried out in 47 Italian coronary care units.
Patients and methods: 757 patients from the GISSI-3 echo substudy population with their first acute myocardial infarct were studied by echocardiography at 24–48 hours from symptom onset (S1), at discharge (S2), at six weeks (S3), and at six months (S4). The diagnosis of left ventricular thrombosis was based on the detection of an echo dense mass with defined margins visible throughout the cardiac cycle in at least two orthogonal views.
Results: In 64 patients (8%), left ventricular thrombosis was detected in one or more examinations. Compared with the remaining 693 patients, subjects with left ventricular thrombosis were older (mean (SD) age: 64.6 (13.0) v 59.8 (11.7) years, p < 0.005), and had larger infarcts (extent of wall motion asynergy: 40.9 (11.5)% v 24.9 (14)%, p < 0.001), greater depression of left ventricular ejection fraction at S1 (43.3 (6.9)% v 48.1 (6.8)%, p < 0.001), and greater left ventricular volumes at S1 (end diastolic volume: 87 (22) v 78 (18) ml/m2, p < 0.001; end systolic volume: 50 (17) v 41 (14) ml/m2, p < 0.001). The prevalence of moderate to severe mitral regurgitation on colour Doppler at S1 was greater in patients who had left ventricular thrombosis at any time (10.2% v 4.2%, p < 0.05). On stepwise multiple logistic regression analysis the only independent variables related to the presence of left ventricular thrombosis were the extent of wall motion asynergy and anterior site of infarction.
Conclusions: Left ventricular thrombosis is not reduced, and may even be increased, by early moderate to severe mitral regurgitation after acute myocardial infarction. The only independent determinant of left ventricular thrombosis is the extent of the akinetic-dyskinetic area detected on echocardiography between 24–48 hours from symptom onset.
Keywords: echocardiography, myocardial infarction, thrombosis, mitral valve
Left ventricular thrombosis is a well recognised complication of acute myocardial infarction and represents a possible source of systemic embolism. Clinicopathological studies have shown that left ventricular thrombus formation after an infarct is related to the site and size of the infarct and the degree of pump failure.1,2 Different prevalences of left ventricular thrombosis have been found in patients with anterior or inferior acute myocardial infarcts, ranging from 25–55% in the former and from 2–4% in the latter.3–5 In the GISSI-3 patient population, which was at low to medium risk of left ventricular thrombosis, Chiarella and colleagues reported a 5.1% predischarge prevalence of ventricular thrombi, with the highest occurrence rate among patients with anterior acute myocardial infarction and an ejection fraction of less than 40%.6 In that study, both Killip class > 1 and early intravenous β blocker treatment were independently associated with a greater likelihood of left ventricular thrombus formation before discharge after an anterior acute myocardial infarct. Recently, it has been reported that abnormal left ventricular flow patterns in the setting of acute myocardial infarction are strongly associated with thrombus formation.7,8
A protective role of severe mitral regurgitation against left ventricular thrombosis has been shown recently in patients with dilated cardiomyopathy.9 The protective effect of mitral regurgitation may be a result of increased early diastolic inflow velocities at the mitral annulus or through the entire length of the left ventricle.10,11 Few data from prospective studies are available on the effect of mitral regurgitation on left ventricular thrombus formation in patients with acute myocardial infarction.12
Our aims in this study were to use cross sectional and colour Doppler echocardiography—which are widely employed, non-invasive techniques for assessing mitral regurgitation and left ventricular thrombus formation13,14—to determine the prevalence and correlates of left ventricular thrombosis in a sample of patients with acute myocardial infarction; and also to determine whether early mitral regurgitation (within 48 hours of acute myocardial infarction), with increased inflow velocities, is protective against left ventricular thrombus formation.
METHODS
The GISSI-3 echo substudy initially evaluated a subset of 925 patients (from 47 coronary care units) from among the 19 394 patients randomised within the GISSI-3 trial.15 Forty seven patients (5%) did not have confirmed acute myocardial infarction and were excluded, while the remaining 878 (95%) with echocardiographic recordings suitable for qualitative and quantitative analysis were enrolled in the study. The protocol required serial standard ECG and echocardiographic studies at 24–48 hours from symptom onset (S1; mean (SD), 36 (8) hours); at hospital discharge (S2; 12 (4) days); at six weeks (S3; 48 (9) days); and at six months (S4; 194 (17) days) after acute myocardial infarction.
Echocardiographic study
Echocardiographic scans were obtained using commercially available instruments. Images were recorded on 0.5 inch VHS videotapes to allow real time and slow motion playback review and quantitative analysis. In each patient, multiple views were obtained in the parasternal long and short axis, apical four chamber, apical two chamber, and subcostal long and short axis planes. Short axis views were recorded at basal (mitral valve level), middle (papillary muscle level), and apical positions.
All cross sectional echocardiograms were submitted to the core laboratory at the Research Centre of the National Association of Hospital Cardiologists (Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO)) in Florence for an assessment of technical quality and suitability for quantitative analysis. Scans were considered acceptable if they allowed visualisation of all myocardial segments from at least two complementary or orthogonal views, and visual assessment of both endocardial motion and myocardial thickening. For wall motion analysis a 16 segment model was used, and each myocardial segment was scored using the semiquantitative grading system proposed by the American Society of Echocardiography (1, normal; 2, hypokinetic; 3, akinetic; 4, dyskinetic; 5, aneurysmal).16
Videotape analysis was undertaken centrally by three expert investigators unaware of patients' clinical, ECG, or angiographic data, who assigned the wall motion score by consensus. The percentage wall motion abnormality—an index of the extent of ischaemic myocardial damage—was obtained by dividing the number of akinetic, dyskinetic, and aneurysmal segments by the total number of segments evaluated. Previously reported values for our interobserver and intraobserver reproducibility in assessing the wall motion score were 89% and 93%, respectively.17
Echocardiographic images were then transferred to the hard disk of a Tomtec–Freeland Medical imaging off-line computer analysis system, where they were digitised to obtain endocardial contours and left ventricular cavity areas at end diastole and end systole from two apical orthogonal views. The modified Simpson rule was used to obtain biplane left ventricular volumes, and the ejection fraction was derived from the standard equation. All measurements were made by a single experienced operator (PLT) from three cardiac cycles, and the mean value was considered for analysis and corrected for body surface area to obtain the volume index. Intraobserver variability in the quantitative evaluation of end diastolic and end systolic volumes was 2.6 (2)% and 3.7 (3)%, respectively.18
Colour Doppler evaluation of mitral regurgitation was qualitative for the presence/absence of regurgitation and semiquantitative for estimation of severity. Three grades of severity—mild, moderate, and severe—were identified, according to previously reported criteria, taking into account the width and depth of regurgitant jets from different views.19 Regurgitation was evaluated as mild (score grade 1) if the jet width was judged visually to be less than or equal to one third of the receiving chamber width; moderate (grade 2) if the jet width was visually judged to be more than one third and less than half of the receiving chamber width; and severe (grade 3) if the jet width was half or more than half of the receiving chamber width. The relation between jet size and receiving chamber size was assessed by visual integration of the information obtained from all the different transducer positions, to ensure exploration of the entire receiving chamber and to allow adequate evaluation of the three dimensional characteristics of the jet. In our laboratory, the concordance between two observers for semiquantitative assessment of mitral regurgitation is 98%.20
Left ventricular thrombus was defined by cross sectional echocardiography as an echogenic mass with well defined margins, adjacent to an asynergic myocardial segment, easily distinguishable from other cardiac structures such as anomalous left ventricular chords or trabeculations. It had to be seen in more than one view and to be visible throughout the cardiac cycle.21,22
Diastolic flow velocity at the left ventricular inflow tract was determined using pulsed Doppler echocardiography in the apical four chamber view. The Doppler sample volume was placed about 1 cm below the plane of the mitral annulus between the tips of the mitral leaflets, where maximum flow velocity in early diastole was recorded.23 The following variables were measured: peak flow velocity in early diastole (E), peak flow velocity at atrial contraction (A), peak E/A wave velocity ratio, and the deceleration time of early filling. The mean of three consecutive beats was used for each measurement.
Left atrial area was calculated at end systole, in the apical four chamber view, tracing the inner border of the endocardial echoes of the chamber walls, and using the plane of the mitral annulus as the inferior boundary. The mean of three consecutive beats was used for each measurement.24
Statistics
Statistical analyses were undertaken using a 6.04 version of SAS software for PC. Comparisons between groups were made using an unpaired Student t test and χ2 analysis for continuous and categorical variables, respectively. Stepwise multivariate logistic regression was used to identify independent predictors of thrombus formation. A probability value of p < 0.05 was considered significant. Values are given as mean (SD).
RESULTS
Of 878 patients enrolled in the GISSI-3 echo substudy, 757 with their first acute myocardial infarct and with available echocardiographic recordings at S1, S2, S3, and S4 were available for analysis. Moderate or severe mitral regurgitation was found in 36 patients (4.7%) at S1. Wall motion abnormality (34.1 (12.7)% v 25.8 (14.4)%; p < 0.001), peak E velocity (73 (17) v 65 (22) cm/s; p < 0.01), and left atrial area (18.5 (3.5) v 17.1 (3.7) cm2; p < 0.05) were increased in patients with moderate/severe mitral regurgitation compared with those with mild/absent mitral regurgitation. There was no difference between the two groups in left ventricular end diastolic volume indexed for body surface area (80.1 (18.7) v 78.8 (18.9) ml/m2), left end systolic volume (43.6 (14.0) v 41.6 (14.3) ml/m2), and left ventricular ejection fraction (46 (6)% v 47 (7)%).
Left ventricular thrombus formation was detected in 64 patients (8%) in one or more of the four consecutive examinations. In 49 patients (77%), the thrombosis was recognised during the hospital admission, while thrombosis of new onset was found in only 15 patients (23%) during the six month follow up period. The clinical and echocardiographic characteristics of subjects with and without left ventricular thrombosis at any time are shown in table 1. Compared with the remaining 693 patients, subjects with left ventricular thrombosis were significantly older (p < 0.005), more often had an anterior infarct on ECG (p < 0.001), and had a greater percentage of wall motion abnormality (p < 0.001). In addition, they showed significantly greater end diastolic and end systolic volumes and a more depressed left ventricular ejection fraction on admission.
Table 1.
Clinical and echocardiographic characteristics of patients with acute myocardial infarction according to the presence or absence of left ventricular thrombosis at any time
| LVT absent (n=693) | LVT present (n=64) | p Value | |
| Age (years) | 60 (12) | 64 (13) | <0.005 |
| Female | 17 | 23 | NS |
| Anterior AMI | 27 | 67 | <0.0001 |
| Thrombolysis | 73 | 70 | NS |
| Aspirin | 86 | 80 | NS |
| β Blockers | 27 | 36 | NS |
| Oral anticoagulant | 4.8 | 8.5 | NS |
| PTCA | 4.8 | – | <0.05 |
| CABG | 3.4 | 0.5 | <0.05 |
| Mean LV EF | 48% | 43% | <0.001 |
| LV EDVI (ml/m2) | 78 (12) | 87 (22) | <0.001 |
| LV ESVI (ml/m2) | 41 (14) | 50 (17) | <0.001 |
| LV WMA (%) | 25 (14) | 41 (11) | <0.001 |
| MR 2–3 at S1 | 4.2 | 10.9 | <0.05 |
| Peak E velocity (cm/s) | 66 (22) | 62 (27) | NS |
| Left atrial area (cm2) | 17.2 (3.7) | 16.9 (3.8) | NS |
Values are mean (SD) or %.
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; EDVI, end diastolic volume indexed; EF, ejection fraction; ESVI, end systolic volume indexed; LV, left ventricular; LVT, left ventricular thrombus formation; MR, mitral regurgitation (the numbers 2–3 indicate the severity of the regurgitation); PTCA, percutaneous transluminal coronary angioplasty; WMA, wall motion asynergy.
The use of thrombolytic agents, aspirin, and oral anticoagulants did not differ between patients with and without left ventricular thrombi. The prevalence of moderate to severe mitral regurgitation at S1 was greater in patients with left ventricular thrombosis (10.3% v 4.2%, p < 0.005).
During the six month follow up period, the percentage of patients undergoing revascularisation procedures was greater in the group without left ventricular thrombosis (percutaneous transluminal coronary angioplasty: 4.8% v 0%, p < 0.05; coronary artery bypass grafting: 3.4% v 0.5%, p < 0.05).
In a stepwise multiple logistic regression analysis, the percentage of wall motion abnormality (odds ratio (OR) 1.07, 95% confidence interval (CI) 1.05 to 1.19) and anterior site of infarction (OR 2.32, 95% CI 1.26 to 4.26) were the only independent correlates of left ventricular thrombosis at any time. Moderate to severe mitral regurgitation at S1 or at any time thereafter had no significant effect on left ventricular thrombosis.
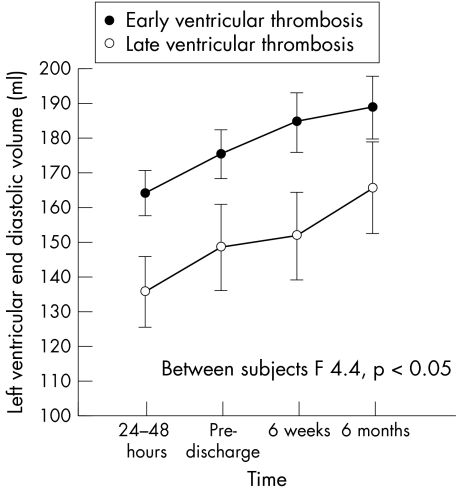
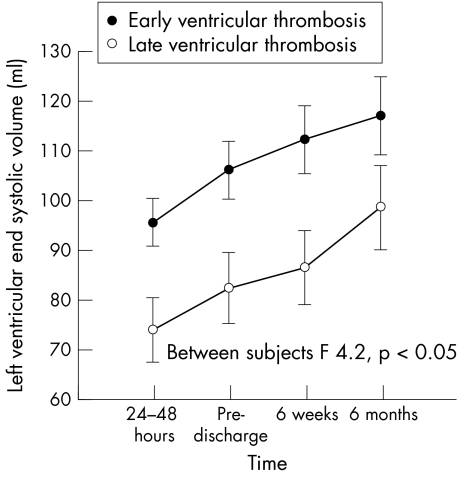
The patients were subdivided in two groups according to the presence of left ventricular thrombosis before (early) or after discharge (late). Both groups showed progressive left ventricular enlargement, but at all times the left ventricular volumes were significantly greater in the group with early thrombus formation (figs 1 and 2). No difference was found between the groups for left ventricular ejection fraction or wall motion abnormality.
Figure 1.
End diastolic left ventricular volume in patients with early and late ventricular thrombus formation.
Figure 2.
End systolic left ventricular volume in patients with early and late ventricular thrombus formation.
When compared with the 693 patients without left ventricular thrombus formation, those with late left ventricular thrombosis had a greater degree of wall motion abnormality (41 (11)% v 25 (11)%; p < 0.001), but there was no difference in left ventricular volume or ejection fraction.
In a subgroup of 74 patients considered to be at increased risk of left ventricular thrombosis on the basis of an anterior infarct on ECG and ≥ 40% wall motion abnormality, a left ventricular thrombus was present in 20 (27%). In comparison with the remaining 54 subjects who did not have left ventricular thrombosis, these 20 patients were older (70 (11) v 61 (12) years, p < 0.005) but were not more likely to have moderate to severe mitral regurgitation on colour Doppler at the time of admission (table 2).
Table 2.
Prevalence of grade 2–3/3 mitral regurgitation at onset of symptoms in a subgroup of patients with anterior myocardial infarction and ≥ 40% wall motion abnormality
| LVT absent (n=54) | LVT present (n=20) | p Value | |
| Age (years) (mean (SD)) | 61 (12) | 70 (11) | <0.05 |
| 2–3/3 MR (%) | 43 | 57 | NS |
LVT, left ventricular thrombus formation; MR, mitral regurgitation.
DISCUSSION
Left ventricular thrombosis in acute myocardial infarction
The prevalence of left ventricular thrombosis, a well recognised complication of acute myocardial infarction, differs in various published reports.3–5 Discrepancies may reflect differences in patient selection, the timing of examinations, and drug treatments used.
The present study, involving a prospective serial echocardiographic evaluation over six months, allowed us to assess the incidence of left ventricular thrombosis in a population of patients with their first acute myocardial infarction who were treated according to recent therapeutic protocols.15 The occurrence of left ventricular thrombosis at any time was 8%—lower than previously reported3–5 but very close to the value of 5.1% found in the whole GISSI-3 population on their predischarge echocardiographic evaluation.6 This lower left ventricular thrombosis rate probably reflects patient selection, as subjects in shock or with haemodynamic instability were excluded from our study population. Furthermore, our protocol included only those patients who had echocardiographic examinations suitable for qualitative and quantitative analyses, which introduced another a priori selection bias. The slightly higher left ventricular thrombosis rate compared with the Chiarella GISSI-3 data6 reflects differences in the study protocols: while the GISSI-3 predischarge echocardiogram provides only one target time in the left ventricular thrombosis history, our protocol—with serial echocardiographic recordings—allowed us to evaluate the phenomenon over time. Furthermore, the early echocardiographic examination performed at S1 in our study (36 (8) hours from symptom onset) could explain our greater incidence of left ventricular thrombosis, as left ventricular thrombi can occur within a few hours after experimental infarction and disappear later.25–28
The appearance of left ventricular thrombi during acute myocardial infarction has been related to an anterior site of infarction, to deterioration in left ventricular function, and to aneurysm formation.2,26,28,29 Our study confirms these reported relations in a low risk population, in which the extent of wall motion abnormalities and an anterior site of infarction were the only independent correlates of left ventricular thrombus formation at any time. However, in our cohort, late left ventricular thrombus formation was clearly related to the extent of wall motion abnormality, whereas early thrombus formation was related to left ventricular volume: patients with early thrombosis had larger volumes than those with late thrombosis or subjects with no thrombosis. This suggests a possible additional role of left ventricular remodelling in causing early left ventricular thrombosis.
Despite its widespread use in the clinical setting, the effect of thrombolysis on left ventricular thrombus formation during acute myocardial infarction remains unclear. Thrombolytic agents should protect patients with myocardial infarcts from left ventricular thrombi as their use reduces blood coagulability, lessens the infarcted area, and results in improvement in left ventricular function. Nevertheless, there is no agreement on the effectiveness of systemic thrombolysis in preventing left ventricular thrombosis after acute myocardial infarction.30–32 In the present study, the lack of any reduction in left ventricular thrombus formation in patients receiving thrombolytic treatment may be explained by the rather small infarct size in our study population.
Relation between mitral regurgitation and left ventricular thrombi in patients with acute myocardial infarction
Previous experimental and clinical studies have shown abnormal blood flow patterns in the left ventricular cavity during acute myocardial infarction. In dogs with experimental infarcts, blood flow patterns depend on the degree of left ventricular asynergy33: in apical dyskinesia, the inflow blood distribution towards the infarcted area is virtually absent, with stagnation at the apex, while in akinesia the blood flow bifurcates into two streams, with stagnant blood between them. Both these abnormal flow patterns are responsible for thrombus formation.33 In a clinical study of patients with acute myocardial infarction, two distinct abnormal left ventricular flow patterns related to the development of thrombus have been described7: a free vortex ring type and an apical rotating type. More recently, Van Dantzig and colleagues confirmed the pathogenic significance of flow disturbance for thrombus formation, stating that abnormal left ventricular flow on admission was the only independent predictor of left ventricular thrombosis after acute myocardial infarction.8
On the basis of the latter observations, in the present study we tested the hypothesis that mitral regurgitation—a condition associated with increased left ventricular inflow velocity and reduced stasis—might prevent left ventricular thrombosis in patients with their first acute myocardial infarction. Our results suggest that in a low risk population with acute myocardial infarction, mitral regurgitation does not prevent left ventricular thrombus formation; indeed we observed an increase in left ventricular thrombosis in patients with significant mitral regurgitation. These findings are consistent with those of Van Dantzig and colleagues,12 who found a progressive increase in left ventricular thrombosis with increasing mitral regurgitation in patients with acute myocardial infarction, though they appear to contradict the findings of other investigators who have shown that mitral regurgitation is protective against left ventricular thrombosis in patients with dilated cardiomyopathy.9,10,34 This discrepancy is probably accounted for by differences in the patient populations—thus subjects with a severely depressed ejection fraction but without acute myocardial infarction are likely to behave differently from low risk patients with acute myocardial infarction and a better preserved left ventricular function.
In patients with mitral regurgitation, peak E velocity seems to correlate with regurgitant fraction and has been proposed as a screening tool for evaluating the severity of mitral regurgitation.35 The higher peak E velocity recorded in our patients who had moderate or severe mitral regurgitation at S1 confirms the presence of increased early filling velocities at mitral annulus level, and possibly at all levels of left ventricular inflow, but this was not sufficient to prevent left ventricular thrombosis. However, we did not evaluate apical flow velocities throughout the cardiac cycle so we could not assess the influence of this variable on left ventricular thrombus formation.
Limitations
A potential limitation of the present study was that we focused on the severity of early mitral regurgitation, while left ventricular thrombosis was assessed in all four echocardiographic studies. The degree of mitral regurgitation may change after acute myocardial infarction, particularly after reperfusion therapy, and such a change may influence the development of left ventricular thrombosis. However, left ventricular thrombosis of new onset was found only in 23% of patients during the six month follow up period, whereas 77% of patients developed left ventricular thrombus during the acute phase of myocardial infarction. When we included moderate to severe mitral regurgitation at any time after the infarct in the multivariate model, the results did not change.
Conclusions
We found that early mitral regurgitation and left ventricular thrombus formation were significantly related to the extent of wall motion abnormality. Both findings may reflect greater myocardial damage during acute myocardial infarction and this could explain the lack of effect of early mitral regurgitation in preventing left ventricular thrombosis. Our data suggest that left ventricular remodelling could be another potentially important determinant in early thrombus formation.
Appendix
The following individuals and institutions participated in the GISSI-3 echo substudy:
Chairman
G L Nicolosi
Core ECO Laboratories
F Gentile, P Giannuzzi, G L Nicolosi, P L Temporelli
Core ECG Laboratories
E Bosimini
Scientific and organising secretariat and data management
A P Maggioni, D Lucci
Participating clinical centres
Barga (M Lunardi, A Azzarelli); Borgosesia (P Devecchi, F Forni,); Cagliari Brotzu (M Putzu, A Pani); Cagliari SS Trinità (L Tocco, W Boi, S Piras); Camposampiero (P Piovesana, F De Conti); Casarano (F De Santis, A Muscella); Castellammare di Stabia (F Russo, N Di Martino); Catania Cannizzaro (G Centamore, A Milazzotto); Cava dei Tirreni (M Agrusta); Chieti SS Annunziata (A Di Pasquale); Cinisello Balsamo (F Gentile, M Ornaghi, E Mangiarotti); Cittadella (A Carrozza); Città di Castello (S Misuri, G Gambarati); Correggio (S Bendinelli, L Lusetti); Cosenza Civile (G Bisignani, O Serafini); Cuneo (F Margaria, G Ugliengo, F Meinardi); Domodossola (M Modica, M Tessitori, F Barba); Firenze Careggi (A Santini); Firenze Torregalli (L Berti, P Stroder, G Casolo); Galatina (G Manca); Garbagnate Milanese (E Cazzani, P Di Lavore, M Civelli); Genova (F Chiarella, S Domenicucci); Ivrea (G Ronzani); Leno (S Perotti, M Bonaglia); Lugo (R Mantovani); Messina Policlinico (S Carerj, P Grimaldi, F Scapellato); Napoli Monaldi (P Caso); Oristano (S Marchi, E Sanna); Penne (D Di Gregorio, A Vacri, L Mantini); Pisa S Chiara (E Cabani, U Conti); Pistoia (A Giomi, A Alfieri, E Balli); Pontedera (A Paci, G Squarcini, M Masini); Pordenone (P Visentin); Ragusa (R Licitra, C Cintolo); Rho (J Heyman); Roma S Eugenio (A Lax); Roma Fatebenefratelli (C Peraldo Neja); Rossano (S Salituri); Sarzana (D Bertoli, F Vivaldi); Scandiano (G Gambarati); Tradate (S Lombroso, S Giani, D Barbieri); Vasto (G Di Marco, G Levantesi, G De Vito, E Bottari); Venezia (L Facchin, G Ramuscello); Viterbo (A Capezzuto); Voghera (P Gandolfi, G Bergognoni).
REFERENCES
- 1.Jordan RA, Miller RD, Edwards JE, et al. Thrombo-embolism in acute and healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952;6:1–6. [DOI] [PubMed] [Google Scholar]
- 2.Asinger RW, Mikell FL, Elsperger J, et al. Incidence of left ventricular thrombosis after acute transmural myocardial infarction. N Engl J Med 1981;305:297–302. [DOI] [PubMed] [Google Scholar]
- 3.Keating EC, Gross SA, Schlamowitz RA. Mural thrombi in myocardial infarction: prospective evaluation by two-dimensional echocardiography. Am J Med 1983;74:989–95. [DOI] [PubMed] [Google Scholar]
- 4.Stratton JR. Mural thrombi of the left ventricle. Chest 1983;83:166–8. [DOI] [PubMed] [Google Scholar]
- 5.Johannessen KA, Nordrehaugh JE, Von der Lippe G. Left ventricular thrombosis and cerebrovascular accident in acute myocardial infarction. Br Heart J 1984;51:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiarella F, Santoro E, Domenicucci S, et al. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998;81:822–7. [DOI] [PubMed] [Google Scholar]
- 7.Delemarre BJ, Visser CA, Bot H, et al. Prediction of apical thrombus formation in acute myocardial infarction based on left ventricular spatial flow pattern. J Am Coll Cardiol 1990;15:355–60. [DOI] [PubMed] [Google Scholar]
- 8.Van Dantzig JM, Delemarre BJ, Bot H, et al. Doppler left ventricular flow pattern versus conventional predictors of left ventricular thrombus after acute myocardial infarction. J Am Coll Cardiol 1995;25:1341–6. [DOI] [PubMed] [Google Scholar]
- 9.Kalaria VG, Passannante MR, Shah T, et al. Effect of mitral regurgitation on left ventricular thrombus formation in dilated cardiomyopathy. Am Heart J 1998;135:215–20. [DOI] [PubMed] [Google Scholar]
- 10.Blondheim DS, Jacobs LE, Kotler MN, et al. Dilated cardiomyopathy with mitral regurgitation: decreased survival despite a low frequency of left ventricular thrombus. Am J Cardiol 1988;122:763–71. [DOI] [PubMed] [Google Scholar]
- 11.Takenaka K, Dabestani A, Gardin JM, et al. Pulsed Doppler echocardiographic study of left ventricular filling in dilated cardiomyopathy. Am J Cardiol 1986;58:143–7. [DOI] [PubMed] [Google Scholar]
- 12.Van Dantzig JM, Delemarre BJ, Bot H, et al. Usefulness of mitral regurgitation in protecting against left ventricular thrombus after acute myocardial infarction. Am J Cardiol 1995;15:1270–2. [PubMed] [Google Scholar]
- 13.Helmcke F, Nanda N, Hsiung MC, et al. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation 1987;75:175–83. [DOI] [PubMed] [Google Scholar]
- 14.Stratton JR, Lighty GW, Pearlman AS, et al. Detection of left ventricular thrombus by two-dimensional echocardiography: sensitivity, specificity, and causes of uncertainty. Circulation 1982;66:156–66. [DOI] [PubMed] [Google Scholar]
- 15.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI-3). Effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet 1994;343:1115–22. [PubMed] [Google Scholar]
- 16.Shiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of left ventricle by two-dimensional echocardiography. American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Ecocardiogr 1989;2:358–67. [DOI] [PubMed] [Google Scholar]
- 17.Badano L, Stoian J, Cervesato E, et al, for the GISSI-3 Echo Substudy Investigators. Reproducibility of wall motion score and its correlation with left ventricular ejection fraction in patients with acute myocardial infarction. Am J Cardiol 1996;78:855–8. [DOI] [PubMed] [Google Scholar]
- 18.Bosimini E, Giannuzzi P, Temporelli PL, et al, for the GISSI-3 Echo Substudy Investigators. Electrocardiographic evolutionary changes and left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 2000;35:127–35. [DOI] [PubMed] [Google Scholar]
- 19.Dall'Aglio V, D'Angelo G, Moro E, et al. Interobserver and echo-angio variability of two-dimensional color Doppler evaluation of aortic and mitral regurgitation. Eur Heart J 1989;10:334–40. [DOI] [PubMed] [Google Scholar]
- 20.Mimo R, Sparacino L, Nicolosi GL, et al. Quantification of mitral regurgitation: Comparison between transthoracic and transesophageal color Doppler flow mapping. Echocardiography 1991;8:619–26. [DOI] [PubMed] [Google Scholar]
- 21.Asinger RW, Mikell FL, Sharma B, et al. Observation on detecting left ventricular thrombus with two-dimensional echocardiography: emphasis on avoidance of false positive diagnosis. Am J Cardiol 1981;47:145–56. [DOI] [PubMed] [Google Scholar]
- 22.Keren A, Billingham ME, Popp RL. Echocardiographic recognition and implications of ventricular hypertrophic trabeculation and aberrant bands. Circulation 1984;70:836–42. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura RA, Abel MD, Hatle LK, et al. Assessment of diastolic function of the heart: background and current application of Doppler echocardiography. Part II. Clinical studies. Mayo Clin Proc 1989;64:181–204. [DOI] [PubMed] [Google Scholar]
- 24.Weyman AE. Left ventricular inflow tract. II. The left atrium, pulmonary veins and coronary sinus. In : Weyman AE, ed. Principles and practice of echocardiography, 2nd ed. Philadelphia: Lea & Febiger, 1994:471–97.
- 25.Domenicucci S, Bellotti P, Chiarella F, et al. Spontaneous morphologic changes of left ventricular thrombus: a prospective two-dimensional echocardiographic study. Circulation 1987;75:737–43. [DOI] [PubMed] [Google Scholar]
- 26.Spirito P, Bellotti P, Chiarella F, et al. Prognostic significance and natural history of left ventricular thrombi in patients with acute myocardial infarction: a two-dimensional echocardiographic study. Circulation 1985:72:774–80. [DOI] [PubMed] [Google Scholar]
- 27.Mikell FL, Ainger RW, Elspherger KJ, et al. Tissue acoustic properties of fresh ventricular thrombi and visualization by two-dimensional echocardiography: experimental observations. Am J Cardiol 1982;49:1157–65. [DOI] [PubMed] [Google Scholar]
- 28.Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the post-hospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990;15:790–800. [DOI] [PubMed] [Google Scholar]
- 29.Neskovic AN, Marinkovic J, Bolic M, et al. Predictors of left ventricular thrombus formation and disappearance after anterior wall myocardial infarction. Eur Heart J 1998;19:908–16. [DOI] [PubMed] [Google Scholar]
- 30.Granbow DW, Valentini VV, Armstrong WF. Thrombolytic therapy and intravenous heparin in acute myocardial infarction do not affect the incidence of left ventricular thrombus formation. Am Heart J 1994;127:1424–6. [DOI] [PubMed] [Google Scholar]
- 31.Lupi G, Domenicucci S, Chiarella F, et al. Influence of thrombolytic treatment followed by full dose anticoagulation on the frequency of left ventricular thrombi in acute myocardial infarction. Am J Cardiol 1989;64:588–90. [DOI] [PubMed] [Google Scholar]
- 32.Pizzetti G, Belotti G, Margonato A, et al. Thrombolytic therapy reduces the incidence of left ventricular thrombus after anterior myocardial infarction. Eur Heart J 1996;17:421–8. [DOI] [PubMed] [Google Scholar]
- 33.Beppu S, Izumi S, Miyatake K, et al. Abnormal blood pathways in left ventricular cavity in acute myocardial infarction: experimental observations with special reference to regional wall motion abnormality and hemostasis. Circulation 1988;78:157–64. [DOI] [PubMed] [Google Scholar]
- 34.Maze SS, Kotler MN, Parry WR. Flow characteristics in the dilated left ventricle with thrombus: qualitative and quantitative Doppler analysis. J Am Coll Cardiol 1989;13:873–81. [DOI] [PubMed] [Google Scholar]
- 35.Thomas L, Foster E, Schiller N. Peak mitral inflow velocity predicts mitral regurgitation severity. J Am Coll Cardiol 1998;31:174–9. [DOI] [PubMed] [Google Scholar]