Accumulating evidence suggests that proinflammatory cytokines such as tumour necrosis factor (TNF) α, interleukin (IL)-1β, and IL-6 are likely to be involved in the pathogenesis of advanced cardiac failure. Cytokine actions directly identified to date include promotion of systemic catabolism, myocardial depression, cardiac hypertrophy, and apoptosis of myocytes in congestive heart failure (CHF).
IL-18, a new member of the IL-1 family, is a proinflammatory cytokine with multiple biologic functions.1 In concert with IL-12, IL-18 stimulates Th1 mediated immune responses; by itself, IL-18 can stimulate Th2 cytokine production. IL-18, originally named as an interferon γ inducing factor (IGIF),2 can induce TNFα and IL-6 in murine macrophages.3 Pomerantz and colleagues demonstrated that IL-18 is expressed in vascular endothelial cells and macrophages in human heart, and that IL-18 binding protein, which is derived from a gene distinct from the IL-18 receptor gene and can neutralise IL-18 actions, reduces human myocardial reperfusion injury after 30 minutes of ischaemia.4,5 We hypothesised that IL-18 might contribute to immune activation and cardiac dysfunction in congestive heart failure. In this study we examined serum concentrations of IL-18 in patients with CHF to examine whether the cytokine was involved in the pathophysiology of this syndrome.
METHODS
Subjects included 34 consecutively recruited patients (20 men and 14 women aged 42–83 years, mean 64 years) who had chronic, stable symptomatic heart failure representing New York Heart Association (NYHA) functional class II–IV for more than two months. The cause of heart failure was dilated cardiomyopathy (DCM) in 20 patients, and old myocardial infarction (OMI; more than three months previously) in 14 patients. Seventeen patients were classified according to the standards of the NYHA as functional class II, 14 patients as class III, and three patients as class IV. No significant differences were evident between patients in different functional classes with respect to age, sex, or left ventricular ejection fraction (data not shown). Patients with significant concomitant diseases such as infections, renal failure, cancer, or autoimmune diseases were excluded. In 14 consecutively recruited patients with stable angina pectoris without CHF (SAP) who also were studied, the diagnosis of angina was based on: a history of chest pain on exercise; documented ST depression with chest pain in a treadmill exercise test; angiographically proven stenosis causing narrowing of diameter exceeding 50% in at least one major epicardial coronary artery; and absence of segmental asynergy in biplanar left ventriculography. As controls, we included 10 healthy subjects who had no evidence of ischemic heart disease and normal physical examinations, resting ECG, and echocardiograms. All patients gave informed consent in advance for their participation, and the ethics committee at our institution approved the protocol.
Fasting blood samples were collected in the morning after rest in the supine position for 20 minutes. Blood was withdrawn from an antecubital vein into non-heparinised tubes and kept on ice, and then centrifuged at 1710 g for 15 minutes at 4°C. Immediately after centrifugation, serum samples were stored at −80°C until they were assayed. Concentrations of TNFα (lower limit of detectability, 0.5 pg/ml) and IL-6 (lower limit of detectability, 0.2 pg/ml) were determined by enzyme linked immunosorbent assays (QuantiGlo, R&D Systems, Minneapolis, Minnesota, USA), as were concentrations of IL-18 (lower limit of detectability, 25.6 pg/ml) (MBL, Nagoya, Japan). Left ventricular measurements were obtained from standard two dimensional and M mode echocardiography.
Data are expressed as mean (SEM). Analysis of variance (Kruskal-Wallis test, followed by the Mann-Whitney U test) was used for statistical comparisons. Spearman’s rank correlation test was used for correlations. A value of p < 0.05 was considered to indicate significance.
RESULTS
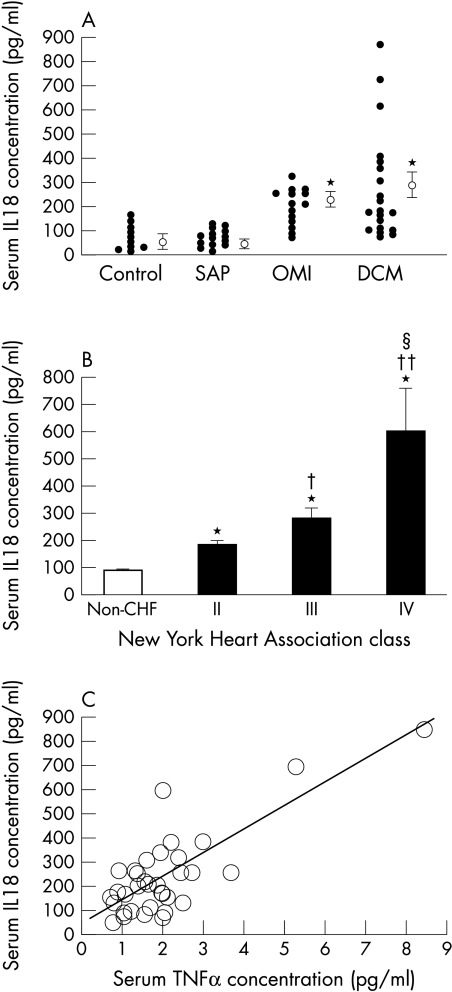
No significant difference in age, heart rate, or systolic blood pressure was present between patient groups. However, the mean ejection fraction was significantly lower in CHF patients (DCM or OMI) than in SAP or control subjects (table 1). Serum IL-18 concentrations did not differ significantly between SAP and controls (81 (9.1) v 86 (18) pg/ml) (fig 1A). Serum IL-18 concentrations in CHF patients were significantly higher than in non-CHF subjects (CHF v non-CHF, 255 (30) v 83 (9.0) pg/ml, p < 0.001). Serum IL-18 concentrations in the DCM group and the OMI group did not differ significantly (284 (49) v 212 (20) pg/ml). Serum TNFα concentrations also were higher in patients with CHF than in non-CHF subjects (1.9 (0.3) v 1.1 (0.1) pg/ml, p < 0.05).
Table 1.
Characteristics of study population
| DCM | OMI | SAP | Control subjects | |
| Age (years) | 61(3) | 67(2) | 67(2) | 67(2) |
| Men/women | 13/7 | 7/7 | 11/3 | 4/6 |
| LVEF (%) | 29(2)* | 30(2)* | 67(2) | 69(3) |
| LVDd (mm) | 65(2)* | 61(2)* | 46(2) | 45(1) |
| SBP (mm Hg) | 116(3) | 120(5) | 128(5) | 126(4) |
| HR (beats/min) | 68(2) | 71(3) | 62(2) | 72(3) |
| Serum creatinine (mg/dl) | 1.1(0.1) | 0.9(0.1) | 0.9(0.1) | 0.9(0.1) |
| BMI (kg/m2) | 20.8(0.8) | 22.6(0.9) | 23.7(0.8) | 23.4(0.8) |
| Hb (g/dl) | 12.8(0.5) | 13.3(0.4) | 12.7(0.3) | 13.4(0.4) |
| Medication (n (%)) | ||||
| Diuretics | 19 (95) | 11 (79) | ||
| Digitalis | 11 (55) | 8 (57) | ||
| ACEI/ARB | 16 (80) | 9 (64) | 3(21) | |
| β Blockers | 3 (15) | 1 (5) | 3(21) | |
Data presented are mean (SEM), or number of patients.
*p<0.001v SAP or control.
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; DCM, patients with congestive heart failure caused by dilated cardiomyopathy; Hb, haemoglobin; HR, heart rate; LVDd, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; OMI, patients with congestive heart failure caused by old myocardial infarction; SAP, patients of stable angina pectoris without congestive heart failure; SBP, systolic blood pressure.
Figure 1.
(A) Scatter plots showing serum concentrations of IL-18 in patients with congestive heart failure caused by dilated cardiomyopathy (n = 20) and old myocardial infarction (n = 14), and patients with stable angina pectoris in the absence of congestive heart failure (n = 14), as well as in age matched control subjects (n = 10). The mean IL-18 concentration (SEM) in each group is shown to the right of each scatter plot. *p< 0.01 compared with control subjects or angina subjects. DCM, patients with congestive heart failure caused by dilated cardiomyopathy; OMI, patients with congestive heart failure caused by old myocardial infarction; SAP, patients of stable angina pectoris without congestive heart failure. (B) Serum concentrations of IL-18 in 34 patients with congestive heart failure and 24 subjects without congestive heart failure (non-CHF) as a function of severity of symptoms according to New York Heart Association (NYHA) functional class (class II, n = 17; class III, n = 14; class IV, n = 3). *p < 0.01 v non-CHF; †p < 0.05 v NYHA II; ††p < 0.01 v NYHA II; and §p < 0.01 v NYHA III. Kruskal-Wallis test results were p < 0.01 when comparing all four groups of subjects. (C) The relation between serum IL-18 and TNFα in patients with congestive heart failure. Serum IL-18 correlated significantly with TNFα (r = 0.785, p < 0.01, n = 34).
Serum IL-18 concentrations increased according to worsening functional class (fig 1B), as did TNFα (data not shown). Serum IL-6 was not related to functional class in our subjects, and no correlation was observed between serum IL-18 and IL-6 concentrations. IL-18 concentration did not significantly correlate with left ventricular end diastolic dimension, left ventricular ejection fraction, or concentrations of natriuretic peptides (data not shown). Serum IL-18 also did not correlate with markers of cachexia such as haemoglobin or body mass index (data not shown). However, a significant correlation was seen between serum IL-18 and TNFα concentrations in patients with CHF (fig 1C).
DISCUSSION
Because serum IL-18 concentration was significantly related to serum TNFα concentration and NYHA class, increased IL-18 release may trigger the elevation in TNFα and modulate the symptoms of CHF. However, the mechanisms of IL-18 elevation and pathophysiologic roles of increased serum IL-18 concentration remain to be elucidated. Discrepancies concerning IL-18 concentrations and cardiac function or cachexia might indicate that IL-18 does not play an important role in CHF. However, varying concentrations of circulating IL-18 binding proteins might mask the correlation between serum IL-18 and other parameters.
In conclusion, we showed for the first time that serum IL-18 was raised in CHF patients, in whom elevations correlated with poorer cardiac functional class and higher TNFα concentrations. IL-18 appears likely to participate in the pathophysiology of CHF, but its specific functional action and possible clinical implications remain to be clarified.
Abbreviations
CHF, congestive heart failure
DCM, dilated cardiomyopathy
IGIF, interferon γ inducing factor
IL, interleukin
OMI, old myocardial infarction
SAP, stable angina pectoris without CHF
TNF, tumour necrosis factor
REFERENCES
- 1.Nakanishi K, Yoshimoto T, Tsutsui H, et al. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 2001;19:423–74. [DOI] [PubMed] [Google Scholar]
- 2.Okamura H, Tsutsi T, Komatsu T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 1995;378:88–91. [DOI] [PubMed] [Google Scholar]
- 3.Netea MG, Kullberg BJ, Verschueren I, et al. Interleukin-18 induces production of proinflammatory cytokines in mice: no intermediate role for the cytokines of the tumor necrosis factor family and interleukin-1β. Eur J Immunol 2000;30:3057–60. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Eisenstein M, Reznikov L, et al. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci 2000;97:1190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pomerantz BJ, Reznikov LL, Harken AH, et al. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1β. Proc Natl Acad Sci 2001;98:2871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]