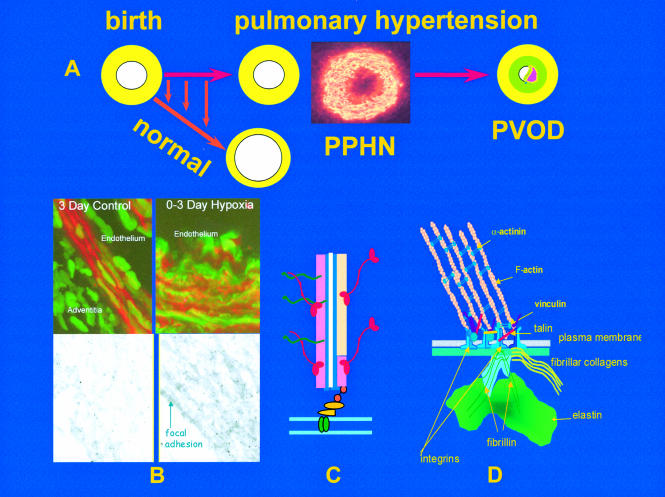
Figure 1.
The upper figure (A) illustrates the rapid reduction in pulmonary arterial wall thickness occurring immediately after birth in the normal lung. This process is profoundly disturbed in persistent pulmonary hypertension of the newborn (PPHN) and an increase in medial thickness eventually leads to pulmonary vascular obstructive disease (PVOD) if the pressure remains high. Insert shows abnormal, hypertensive human peripheral pulmonary artery at three days, stained for γ actin. Mechanisms are illustrated in B, C, and D. (B) Confocal and transmission electron microscopy shows, in the left hand panel, the normal porcine peripheral pulmonary artery, and in the right hand panel, the pulmonary hypertensive vessel at three days. Normal remodelling entails reorganisation of the smooth muscle cell actin cytoskeleton which undergoes transient disassembly as the cells thin and elongate to spread around an enlarging lumen. In PPHN larger cells are packed with red actin myofilaments (phalloidin stained) while immuno-electron microcopy reveals sheets of actin bundles labelled with gold particles rather than actin being diffusely distributed in the cytosol. (C) Illustration of a myofilament. (D) All aspects of vessel wall remodelling are disturbed in PPHN, from the excessive myofilament assembly within the cell, focal adhesion remodelling at the membrane, and excessive connective tissue deposition in the matrix. Gene expression of tropoelastin and type I procollagen is abnormally high and steady state protein concentrations are increased. Vessels appear to become fixed in an incompletely dilated state. This is the beginning of PVOD in the young if the process cannot be arrested therapeutically.