Abstract
A 53 year old woman was referred for percutaneous coronary intervention because of a recent inferior myocardial infarction. During right coronary artery stent implantation, intermittent occlusion of the coronary side branch for the sinus node occurred, associated with intermittent sinus arrest and junctional escape rhythm. This led to speculation about the potential mechanisms for sinus node dysfunction. Degenerative fibrosis of nodal tissue is actually considered the most common cause of bradyarrhythmias. Yet, in everyday practice, no particular attention is usually paid to other potential pathogenic mechanisms such as coronary artery disease. This may be particularly true for elderly patients or patients with multiple risk factors. Thus, sinus node dysfunction may be an unrecognised marker of coronary artery disease.
Ischaemia of the sinus node (SN) is generally considered to be an uncommon pathogenic mechanism of SN dysfunction causing rhythm disturbances. Indeed, so far, direct evidence of an ischaemic insult to the SN has seldom been reported. We had the opportunity to document angiographically this phenomenon in a patient undergoing right coronary artery (RCA) stent implantation.
CASE REPORT
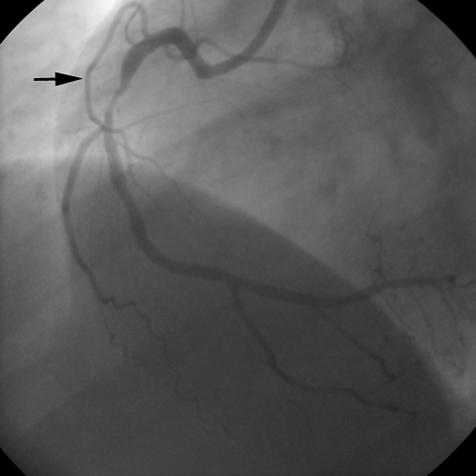
A 53 year old woman with hypertension and hypercholesterolaemia was referred to our institution because of a recent inferior myocardial infarction. Predischarge exercise testing had shown significant ST segment depression associated with angina at a low work load. Coronary angiography showed a critical stenosis in the middle tract of the RCA and a remarkable SN artery originating from the proximal tract of the vessel (fig 1).
Figure 1.
Basal right coronary angiogram showing a critical stenosis in the middle tract of the vessel. The arrow indicates the sinus node artery originating from the proximal tract of right coronary artery.
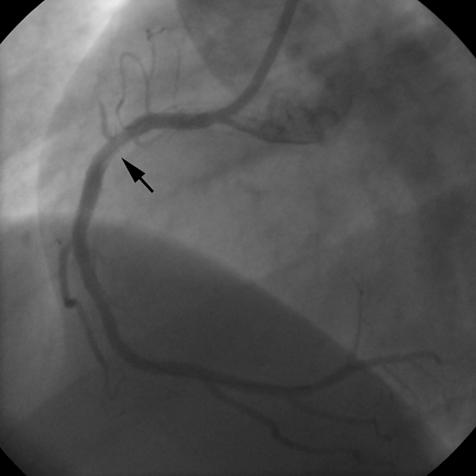
The percutaneous procedure was performed according to standard technique. Immediately after direct stent implantation, a diffuse haziness appeared in the proximal tract of the RCA, close to the proximal edge of the stent, followed by total occlusion of the atrial side branch directed to the SN (fig 2). ECG showed junctional escape rhythm with P wave retroconduction at a heart rate of 50 beats/min, suggesting SN ischaemic arrest. Despite repeated boluses of intracoronary glyceryl trinitrate and intravenous atropine, sinus rhythm did not re-emerge. Intravenous abciximab at conventional doses was then administered. Repeated contrast injections showed slow progression of dye into the sinus branch. TIMI (thrombolysis in myocardial infarction) flow 2–3 in the SN artery was definitely achieved over 10 minutes. The patient remained completely asymptomatic. In the following 12 hours ECG monitoring showed sinus rhythm alternating with junctional escape rhythm.
Figure 2.
Right coronary angiogram after stent deployment. Total occlusion of the sinus node artery is evident. The arrow indicates haziness in the proximal tract of the right coronary artery.
The day after the procedure the patient was in stable sinus rhythm and a slight increase in cardiac markers (creatine kinase MB 25 U/l and cardiac troponin I 2.14 ng/ml) was detected. Two days after the procedure she was discharged and at 7 and 30 days’ follow up she was still asymptomatic and in stable sinus rhythm.
DISCUSSION
In the present clinical case the fortuitous intermittent occlusion of the coronary branch for the SN allowed us to speculate about the potential mechanisms of SN dysfunction.
The degenerative fibrosis of the SN and the atrioventricular node is actually considered to be the most common, if not the only, cause of sick sinus syndrome and atrioventricular block. Indeed, in most patients structural modifications account for an age related decrease of the intrinsic SN discharge rate.1 The organisation of collagen in the SN gradually changes from coarse strands between clusters of nodal cells into a fine web of isolated fibres surrounding each nodal cell.2 SN cells atrophy occurs and SN volume is reduced, with a qualitative, but not quantitative, change in the connective tissue.3
In everyday practice, no particular attention is usually paid to other potential pathogenic mechanisms such as coronary artery disease (CAD). Yet an ischaemic mechanism may well be the cause of SN dysfunction. Most older patients with symptomatic bradyarrhythmias have multiple risk factors for CAD. A recent study evaluated the incidence of coexistent CAD in Chinese people with symptomatic bradyarrhythmias and its relation to coronary risk factors.4 The incidence of angiographic CAD involving node related arteries was quite high (9%). Furthermore, in a postmortem angiographic study in patients with chronic SN dysfunction,5 CAD was relatively common, although no significant lesion obstructing blood flow to the SN was seen in the majority of patients. These studies suggest that among patients with sick sinus syndrome CAD is probably more frequent than believed and that SN ischaemia is an underestimated contributor to SN dysfunction.
SN dysfunction, in patients with inferior myocardial infarction and isolated proximal RCA disease, is related to a reduced flow to the SN artery.6 Clinical studies have shown the relation between ischaemia and SN dysfunction,7 and, accordingly, deliberate SN artery occlusion has been suggested as a potential treatment of chronic inappropriate sinus tachycardia.8
Our case provides further evidence for the ischaemic aetiology of SN dysfunction, emphasised by the intermittent occlusion of the SN branch associated with alternating sinus and escape rhythm. The cause of SN artery occlusion is uncertain in our patient. More than 50% of coronary side branches are at risk for iatrogenic occlusion during percutaneous coronary intervention, because they originate either from the lesion segment itself or from its immediate vicinity, so that they are subjected to temporary occlusion during balloon inflation. Periprocedural myocardial infarction occurred in 5–20% of patients in the pre-stent era. Coronary stenting can prevent abrupt vessel closure but side branch occlusion, especially with low reference diameter,9 and microembolisation are still the most frequent factors responsible for myocardial damage after stent implantation.10 In our case, abciximab opened the SN artery and allowed for successful reperfusion and functional recovery of the SN. Thus, although an atheroembolic mechanism was probably involved, it is quite unlikely that atherosclerotic debris embolised into the SN artery, as the culprit lesion was fairly distal to the SN artery. Coronary spasm can be ruled out because high dose nitrates were ineffective in restoring flow in the SN artery. Plaque shift or direct scratching of the diseased endothelium during stent progression may have played a part in the pathogenesis of this event.
Conclusions
The present clinical case highlights the importance of considering an ischaemic aetiology of SN dysfunction when evaluating patients with symptomatic bradyarrhythmias. This may be particularly true for elderly patients or patients with multiple risk factors for CAD. The presence of SN dysfunction, indeed, may be an unrecognised marker of CAD.
Abbreviations
CAD, coronary artery disease
RCA, right coronary artery
SN, sinus node
TIMI, thrombolysis in myocardial infarction
REFERENCES
- 1.de Marneffe M, Gregoire JM, Waterschoot P, et al. The sinus node and the autonomic nervous system in normals and in sick sinus patients. Acta Cardiol 1995;50:291–308. [PubMed] [Google Scholar]
- 2.Alings AM, Abbas RF, Bouman LN. Age-related changes in structure and relative collagen content of the human and feline sinoatrial node: a comparative study. Eur Heart J 1995;16:1655–67. [DOI] [PubMed] [Google Scholar]
- 3.Shiraishi I, Takamatsu T, Minamikawa T, et al. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation 1992;85:2176–84. [DOI] [PubMed] [Google Scholar]
- 4.Hsueh CW, Lee WL, Chen YT, et al. The incidence of coronary artery disease in patients with symptomatic bradyarrhythmias. Jpn Heart J 2001;42:417–23. [DOI] [PubMed] [Google Scholar]
- 5.Shaw DB, Linker NJ, Heaver PA, et al. Chronic sinoatrial disorder (sick sinus syndrome): a possible result of cardiac ischaemia. Br Heart J 1987;58:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangrum JM, DiMarco JP. The evaluation and management of bradycardia. N Engl J Med 2000;342:703–9. [DOI] [PubMed] [Google Scholar]
- 7.Miller TD, Gibbons RJ, Squires RW, et al. Sinus node deceleration during exercise as a marker of significant narrowing of the right coronary artery. Am J Cardiol 1993;71:371–3. [DOI] [PubMed] [Google Scholar]
- 8.de Paola AA, Horowitz LN, Vattimo AC, et al. Sinus node artery occlusion for treatment of chronic nonparoxysmal sinus tachycardia. Am J Cardiol 1992;70:128–30. [DOI] [PubMed] [Google Scholar]
- 9.Poerner TC, Kralev S, Voelker W, et al. Natural history of small and medium-sized side branches after coronary stent implantation. Am Heart J 2002;143:627–35. [DOI] [PubMed] [Google Scholar]
- 10.Califf RM, Abdelmeguid AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol 1998;31:241–51. [DOI] [PubMed] [Google Scholar]