Abstract
Objectives: To develop and test a new concept of the degradation kinetics of newly developed coronary stents consisting of magnesium alloys.
Methods: Design of a coronary stent prototype consisting of the non-commercial magnesium based alloy AE21 (containing 2% aluminium and 1% rare earths) with an expected 50% loss of mass within six months. Eleven domestic pigs underwent coronary implantation of 20 stents (overstretch injury).
Results: No stent caused major problems during implantation or showed signs of initial breakage in the histological evaluation. There were no thromboembolic events. Quantitative angiography at follow up showed a significant (p < 0.01) 40% loss of perfused lumen diameter between days 10 and 35, corresponding to neointima formation seen on histological analysis, and a 25% re-enlargement (p < 0.05) between days 35 and 56 caused by vascular remodelling (based on intravascular ultrasound) resulting from the loss of mechanical integrity of the stent. Inflammation (p < 0.001) and neointimal plaque area (p < 0.05) depended significantly on injury score. Planimetric degradation correlated with time (r = 0.67, p < 0.01).
Conclusion: Vascular implants consisting of magnesium alloy degradable by biocorrosion seem to be a realistic alternative to permanent implants.
Keywords: stents, biodegradation, tissue engineering
Permanent metallic implants are key treatment options in cardiovascular interventions. However, specific drawbacks limit their more widespread use. These limitations include thrombogenicity, permanent physical irritation, mismatches in mechanical behaviour between stented and non-stented vessel areas, long term endothelial dysfunction, inability to adapt to growth, non-permissive or disadvantageous characteristics for later surgical revascularisation, and chronic inflammatory local reactions. Degradable implants offer more physiological repair, reconstitution of local vascular compliance, and a temporary, limited, longitudinal, and radial straightening effect, including the possibility for growth. These implants are “fulfilling the mission and stepping away”1 and may act as a new biomedical tool satisfying the requirements of compatibility and integration.2 However, most biodegradable synthetic polymer stents must have greater bulk to approximate the mechanical performance required in arteries. Many also induce exaggerated acute and chronic inflammatory responses during degradation.3
To address this issue, we developed and tested a new concept of degradation after endovascular implantation of tailored magnesium alloys. We anticipated a more useful combination of mechanical stability over a limited time and complete degradation of the implants.
METHODS
Alloys
Magnesium alloys containing small amounts of aluminium, manganese, zinc, lithium, and rare earth elements were preselected for their mechanical aspects and tested in vitro for degradation kinetics (synthetic seawater, Ringer lactate, and porcine and human serum; calculated stent half lives for different magnesium alloys were between minutes and about half a year) and the potential influences on cell growth and toxicity in human cell cultures (fibroblasts, and endothelial and vascular smooth muscle cells; the biocompatibility of magnesium alloys was high but diminished by the fast release of aluminium). Standardised discs (200 μm thickness, 3 mm diameter) of six magnesium alloys were implanted into subcutaneous pockets of rats for in vivo assessment of inflammatory reaction and hydrogen production. These responses were minimal for the non-commercial magnesium alloy AE21, containing 2% aluminium atoms and 1% rare earth elements (Ce, Pr, Nd). We used this most promising magnesium alloy for an initial coronary animal study mainly because we expected a mass loss of only 50% during half a year.
Stent prototyping
Tubes (wall thickness between 150–200 μm, length 10 mm, outer diameter 2 mm) were drilled from extruded AE21 rods, and stents (mass 4 mg) were cut by means of a femtosecond laser (Laser Zentrum, Hannover, Germany). This preliminary technology for tube production resulted in unequal wall thickness. The surface was left in its original condition after cutting. After sterilisation, stents were stored in an alcoholic solution.
Animal care and preparation
Experiments were performed according to standard guidelines and were in accordance with local regulations as specified by the animal care committee. Eleven domestic pigs (20–25 kg each) were treated with ticlopidine (250 mg/day orally) for two weeks, starting one day before anaesthesia (ketamine 20 mg/kg intramuscularly; xylazine 2 mg/kg intramuscularly). After endotracheal intubation, the pigs received N2O and O2 (2+1) with an additional 0.5–1.5% isoflurane as needed. An 8 French introducer sheath was placed in the right carotid artery by arteriotomy. Heparin sodium 7500 IU and aspirin 250 mg were then administered. An 8 French JL 3.5 SH guiding catheter for the left and an 8 French AL1 SH guiding catheter for the right coronary artery were used. Quantitative coronary angiography (QCA) was performed off line after administration of 0.2 mg glyceryl trinitrate intracoronary in a 30° right anterior oblique and a 40° left anterior oblique view using the guiding catheter as the reference object. Minimal lumen diameter, reference lumen diameter (proximal and distal of the stent), and percentage diameter of stenosis were measured at the time of follow up.
Stent implantation
A total of 20 stents were implanted in the left anterior descending artery (n = 9), the circumflex artery (n = 9), or the right coronary artery (n = 2). In all cases, a coronary segment with a diameter between 2.5–3.5 mm (proximal segments) and without relevant side branches was selected. The right coronary artery was chosen in two cases because we were unable to place the guiding catheter into the left ostium. After manual crimping, stents were placed (based on vessel size) by pairs in separate arteries (one stent per artery; one inflation, 30 s, 10 atm). Curved artery segments were also included. The intended balloon to artery ratio was 1.3:1 (overstretch injury model).4 After a second intracoronary injection of 0.2 mg of glyceryl trinitrate, the angiography was repeated in accordance with standard procedures. The guiding catheter and sheath were removed, the proximal and distal arteriotomies were ligated, and the skin incision was closed.
Follow up procedure
Follow up procedures through the left carotid access were performed before the pigs were killed at 10 days (three pigs, five stents), 35 days (four pigs, seven stents), and 56 days (three pigs, six stents) after implantation. The angiographic images were taken in the same oblique views after 0.2 mg intracoronary injection of glyceryl trinitrate. In addition, at 35 and 56 days’ follow up, intravascular ultrasound (IVUS) studies were performed (30 MHz, motorised pullback 1 mm/s, transducer positioning 10 mm distal to 10 mm proximal stent edges, CVIS, 3.2 French). At 10 days, IVUS could not be performed because the instrument was not available. Two circumflex artery stents could not be examined and two stents were lost with the premature death of one animal.
Vessel preparation and histological examination
Immediately after angiography, the heart was excised, infused briefly with saline, and pressure perfused (140 cm H2O column) through the aortic root after cross clamping for 30 minutes, using Schaffer solution for fixation. These prefixated stent tissue blocks were dissected, polymer fixated (Technovit 9100, Kulzer, Germany), and cut into 10 μm slices for further histological and morphometric assessments including a semiquantitative, strut related inflammatory response.5 The severity of stent (strut) induced injury was graded according to the method developed by Schwartz and colleagues.6 In addition, parts of the hard fixated blocks were polished, photographed, and digitised for planimetric analysis of neointima and of the strut areas that appeared metallic to assess the rate of intramural degradation corresponding to the time dependent loss of strut masses.
Statistical analysis
The data were analysed using SPSS 6.01 (SPSS Inc, Chicago, Illinois, USA). All data were expressed as mean values. IVUS and QCA data were evaluated by using the Mann-Whitney, Kruskal-Wallis, and Wald-Wolfowitz tests for comparative analysis. For histological data Spearman coefficients were computed. Values of p < 0.05 were considered significant.
RESULTS
Procedural success and macroscopic analysis
The stents were implanted successfully. All animals except one survived the scheduled follow up period without any sign of stent thrombosis or other related events. One pig (two stents) died four days after implantation without apparent reason. The necropsy showed a normal pericardium without effusion, a non-infarcted myocardium, and an open, non-thrombosed and completely expanded stent that was covered by a layer of tissue. In all other pigs, the pericardium was opened to access the beating heart. The visible regions of stented coronary arteries showed the expected “oversizing phenomenon” recognisable as a prominent “hilly” stented region; however, in all but one, there was no visible inflammatory pericardial reaction or epipericardial adhesion at these stented regions. In one case (at 35 days’ follow up) there was a haemodynamically irrelevant pericardial effusion and multiple epipericardial adhesions. The subsequent necropsy by a veterinarian found a common pleuropulmonary inflammatory process not related to the stent.
Follow up angiography
All stented vessels were open without any sign of peripheral embolisation or adjacent or in-stent thrombosis. Table 1 summarises the QCA data.
Table 1.
Significant narrowing of lumen between days 10 and 35 and re-enlargement between days 35 and 56 on quantitative coronary angiography
| Time after implantation (days) | Stents/pig | Reference diameter (mm) | Lumen diameter (mm) | Lumen/ reference | Difference lumen − reference (mm) |
| 10 | 5/3 | 3.36 | 3.46 | 1.04 | +0.1 |
| 35 | 7/4 | 2.59 | 1.63 | 0.63 | −0.95 |
| 56 | 6/3 | 2.81 | 2.48 | 0.88 | −0.32 |
| Compared groups | |||||
| 10–35–56 | NS | ‡ | ‡ | † | |
| 10–35 | ns | ** | ** | ** | |
| 10–56 | ns | n.s. | n.s. | n.s. | |
| 35–56 | ns | ** | * | * | |
*p<0.05, **p<0.01, n.s. p>0.05 Mann-Whitney; †p<0.05, ‡p<0.01, NS p>0.05 Kruskal-Wallis; ns p>0.05 Wald-Wolfowitz.
IVUS data
Table 2 summarises the data for the perfused lumen, plaque, and stent areas. These data document significant increases in stent and lumen areas between days 35 and 56 and an insignificant thickening of the neointima.
Table 2.
Remodelling of stented vessels between days 35 and 56 on intravascular ultrasound
| Time after implantation (days) | Number | Stent area (mm2) | Lumen area (mm2) | Neointimal area (mm2) |
| 35 | 6 | 3.28 | 1.87 | 1.41 |
| 56 | 5 | 6.15 | 3.43 | 2.71 |
| * | ** | n.s. |
*p<0.05, **p<0.001, n.s. p>0.05 Mann-Whitney.
Histological analysis
Data provided at different time periods documented a covering cell layer and, usually, an asymmetric neointimal formation. The medial and adventitial layers were inflamed only in the case of strut related fracturing of the lamina elastica interna or by strut placement in the adventitial layer (n = 3). Analogous to non-degradable metallic stents, with the degradable stents, the semiquantitative assessment of strut related inflammation showed a significant relation between injury, inflammation, and plaque area (table 3). Subjectively, the neointimal formation seemed to be more pronounced at a later time, but histological data indicated no significant change.
Table 3.
Spearman coefficients of histological parameter pairs
| Parameter pair | r |
| INFL-NEO | 0.93** |
| INJURY-INFL | 0.85** |
| INJURY-NEO | 0.63* |
| TIME-INFL | 0.61* |
| TIME-INJURY | 0.6* |
*p<0.05; **p< 0.001; all other correlations insignificant. INFL, inflammation score; INJURY, injury score; NEO, neointimal plaque area; TIME, time after implantation.
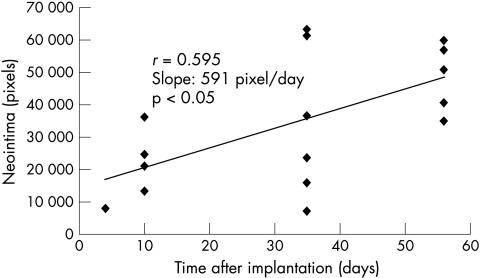
Morphometric assessment of degradation
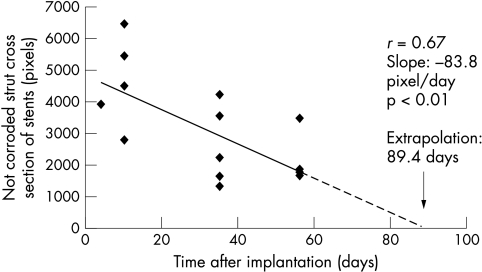
Planimetric data showed a linear increase of intima hyperplasia (fig 1). Figure 2 shows three time points of intravascular degradation (fig 3). Assuming an approximately linear degradation, complete stent degradation should have occurred within 89 days.
Figure 1.
Planimetric analysis showing a linear increase in the neointimal plaque area.
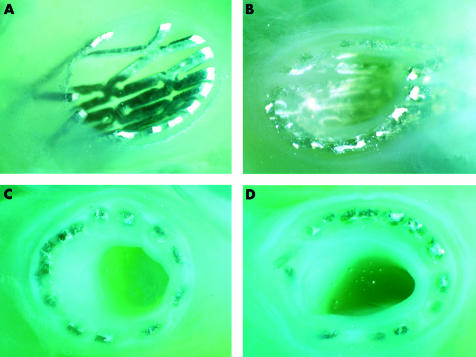
Figure 2.
Four polymer fixated and cut tissue blocks of stents (A) 10, (B) 35, and (C, D) 56 days after implantation. Unequal extension of the stents, and minimal corrosion and neointimal proliferation after 10 days, and advanced and irregular corrosion and proliferation after 35 and 56 days, are seen. The bright areas indicate strut parts that remained metallic.
Figure 3.
Intravascular degradation of magnesium alloy stents in pig coronary arteries. The extrapolated lifetime was calculated under the assumption of a linear disappearance of metallic strut parts.
DISCUSSION
Degradable vascular implants can adapt to growth, allow for late positive remodelling, are optimum vehicles for local drug (or gene) delivery, have a lower risk of late stent thrombosis after brachytherapy, and do not restrict the ability of surgical revascularisation. Most of the reported designs and materials used have resulted in an extensive inflammatory response after implantation in the coronary arteries.3 Only high molecular weight poly-l lactic acid has promised acceptable haemocompatibility and histocompatibility in porcine and human coronary arteries.7,8 In addition, most polymer stents showed an extensive recoil after implantation; because of intrinsically lower strength they need greater bulk to approximate the required mechanical performance.9 This bulkiness may be a limiting factor for application in small vessels such as the coronary arteries. Polymer coated metallic stents may constitute a compromise between mechanical strength and surface properties. However, such hybrids also act as permanent implants and are associated with reported disadvantages.10,11 The use of degradable magnesium alloys leads to electronegative and, therefore, hypothrombogenic surfaces.12 As an essential element,13 slowly degrading magnesium should not harm tissue, particularly since magnesium solutions up to 0.5 mol/l are well tolerated if given parenterally. The mechanical properties and corrosion of magnesium alloys are quite controllable under physiological conditions and match the requirements for degradable implants.14 In body fluids, high chloride concentrations can lead to high mass losses. Attempts to use magnesium in surgery have failed, especially in traumatology, because of hydrogen formation and bacterial infections. As stents are separated from the blood stream by only thin neointimal diffusion barriers we did not expect to encounter a major problem with hydrogen gas formation. Nonetheless, high corrosion rates resulting in serious hydrogen development should be avoided.15 Stroganov (US patent 3,687,135, 1972) described magnesium alloys that contained cadmium; however, no reports on clinical applications are available. We have chosen alloys consisting of non-toxic or low toxic components including some rare earth elements.16 To the best of our knowledge, the present study is the first to describe local responses in coronary arteries in a pig model of biocorrosion. These results are promising and suggest a new, alternative method for hybrid implant degradation that can be combined with local drug delivery principles.
The decision to use the AE21 alloy for our first stent prototypes was based mainly on its degradation kinetics (six month expected half life) and the biocompatibility data from our previous in vitro and in vivo studies. The simple design with its unequal wall thickness caused an unequal expansion and therefore resulted in a different strut related injury relation (fig 2) and a very non-homogeneous injury distribution over the stents. This may explain why we could not histologically verify a significant time course of mainly injury dependent neointima. Nevertheless, this first animal trial of the concept yielded some of the expected results involving thrombogenicity, biocompatibility, and local tissue response during different periods of time.
Thrombogenicity and neoendothelialisation
As expected from theoretical and in vitro data, the thrombogenicity was low. Electrochemical properties of metals are critical for their biological response when placed in contact with blood.12,17 Most metals used for implants are less electronegative than magnesium alloys12 and are therefore more thrombogenic. Another important point for determining haemocompatibility and histocompatibility of a metallic surface is the time needed for neoendothelialisation. In our previous cell culture assessments, the growth of human endothelial cells was not influenced over the 10 day observation period. One pig died four days after implantation. Its stents were opened longitudinally and a closed tissue layer was found over all struts on histological examination. A closed layer was also found in the five stents explanted after 10 days. Thus, when using this alloy, thrombogenicity and neoendothelialisation should not be serious limitations.
Follow up studies
The QCA data (table 1) show a lumen loss from induced plaque formation between days 10 and 35, followed by an increase until day 56 (as the result of positive remodelling). This was documented by IVUS (table 2). IVUS remodelling was further accompanied by an insignificant increase in plaque thickness after day 35 and a significant increase of perfused lumen area. This was made possible by the stent’s loss of mechanical integrity caused by biocorrosion between these two follow up periods. This observation allows us to make two preliminary statements: firstly, the rate of degradation has to be reduced to ensure mechanical integrity for a longer time even with finer devices; and, secondly, the loss of stent integrity allows the local vessel tissue to have a positive remodelling response. However, we must keep in mind that these data are taken from different individual pigs in a follow up involving groups treated according to the same protocol and harvested at different time periods. Serial intraindividual IVUS and QCA data should be used to confirm this observation.
Intravascular and intramural degradation kinetics
The different environmental conditions under the skin and in the vessel wall explain the four times shorter than expected life time. Thus, to define the progression of intramural magnesium alloy degradation it was important to assess sequential strut cross sections. Assuming a linear degradation, the time for a complete breakdown was estimated from the planimetric data (fig 3) to be in the order of three months. Loss of mechanical integrity occurred between days 35 and 56. Some of the metal components released as a result of biocorrosion obviously lead to an inflammatory response and, subsequently, significant neointimal proliferation. It is not known how long a stent needs to remain mechanically stable after placement in a diseased coronary artery. However, mechanical integrity after day 35 may be lost too soon to prevent a secondary vessel occlusion by flaps or by spasm. In addition, our data show clear differences between degradation kinetics in vitro, subcutaneously in vivo, and after intramural vessel placement. The degradation in a pig’s coronary artery wall seems to be accelerated twice as fast as in resting body fluids.
Histological analysis: neointimal proliferation
On the basis of the findings from QCA (table 1), we concluded that there was a significant neointimal proliferation until day 35. Afterwards, the increment was insignificant (IVUS, table 2) until day 56. Planimetry showed a more linear progression (fig 1). As expected, the proliferation process was clearly related to the depth of the local arterial injury caused by the stent struts. When we used our first stent prototypes, some struts caused a very deep medial (and, in three cases, also an adventitial) injury. In addition, the proposed oversizing of the balloons resulted in a partly severe increase of vessel wall tension in the stented and stent adjacent regions. In analogy to the permanent metallic stents, the deep penetration resulted in a more pronounced local neointimal proliferation than did strut elements that did not penetrate the lamina elastica interna (at the same microscopic section, as fig 4 shows). The mean intimal formation was analogous to reported results in comparable pig models with respect to a 28 day period between implantation of permanent metallic stents and harvesting18–21; thus our “raw” prototypes did not induce an extensive neointimal response. We did not find platelet deposition or thrombus formation at the endothelial sites after any of the assessment intervals. For magnesium stents, we expect that thrombus formation and platelet deposition are not dominant factors in stimulating cell proliferation, although this has yet to be proved. Two different processes may be more responsible for neointimal formation: the local tissue “overload” with degradation products resulting from accelerated corrosion; and the dominant relation between strut related injury and induced regional inflammation.
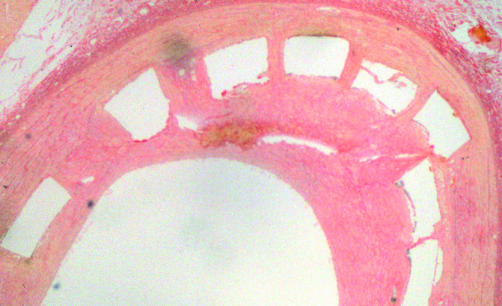
Figure 4.
Histological section of a vessel 35 days after stenting. Deep penetration of the vessel wall results in more neointimal proliferation.
Biocompatibility of degradable biomaterials depends on the solubility of the released degradation products. Their local toxicity is related to the local concentration of the elements over time.22,23 The tissue tolerance for physiologically occurring metals depends on the change of their tissue concentrations induced by corrosion. Thus, elements with high tissue concentrations such as magnesium should have a better biocompatibility during degradation. Changes in local oxygen and pH can be neglected if degradation is slow.2 However, formation of reactive oxygen species through Fenton-type chemical reactions merit more attention, as in the case of non-degradable metals. We chose to use magnesium as the main alloy component because of its surface characteristics and expected local tissue tolerance.
The reason for the observed positive remodelling (table 1, table 2) is unknown. In particular, we must answer the question of whether this host response is a special aspect of bioactivation involved with positive or desirable stimulatory effects24 or whether it is an unspecified response of the pig coronary vessel wall to local inflammation caused by biodegradation and injury. From the data in table 2, we can exclude vessel growth as the main reason for remodelling.
Histological analysis: inflammatory response
Inflammation and neovascularisation are common findings in both experimental animal models and after stenting of human coronary arteries with stainless steel or tantalum stents.19,25,26 In porcine models of oversized stenting, the various degrees of regional vascular injury induce a variable amount of inflammation and neointimal formation. With stainless steel stents, inflammation and injury are equally important for neointimal formation.27 In this respect, on evaluating each strut individually, it was interesting to find negligible inflammatory response or neointimal formation only in the absence of local injury. However, we were not able to separate the contributions of inflammation and injury to neointimal proliferation statistically. Nevertheless, an optimal degradation process should not induce any relevant local inflammatory responses during the degradation period. Slowing down degradation may help to maintain mechanical integrity for a longer time and to keep the release of degradation products low. This should minimise the potential for inflammatory responses.
Limitations
The unpolished bulky prototypes with huge struts result in unequal circumferential mass distributions after inflation in an overstretched pig model. This results in severe vascular injury by superimposing the influence of degradation on inflammation and neointimal formation. The use of filigrane commercial controls did not seem reasonable at this stage of development. To separate the impact of corrosion, an intraindividually matched pair analysis using stainless steel controls with identical design and size is necessary. In this early phase of analysis, we had to accept restrictions in the study design because of technical limitations in prototype production and sterilisation associated with non-commercial magnesium alloys. Another limitation influencing the interpretation of this study is the comparative analysis of different animal groups because of the need to harvest after the three planned study follow up time periods. We gave priority to an estimation of intramural degradation kinetics of magnesium alloys and regional tissue response and confined the assessments to a group analysis.
The excessive weight gain of domestic pigs limited the evaluation period to 56 days. Mini-pigs should be used in further studies to prolong the survival time beyond six months, thus covering a longer period of the life time of the stents. Nevertheless, this study was conceptually important in arriving at significant conclusions, furthering alloy technology and study design, and establishing the biocompatibility and bioreactivity of these new implant materials.
Conclusions
Biocorrosion of magnesium based alloys may be a promising new technology for improving cardiovascular implants as effective temporary systems with inherent or hybrid local drug delivery functions. However, further improvements are necessary with respect to prolongation of the degradation and mechanical stability over a defined time, combined with more sophisticated alloys and design technology. In addition, the short and long term local intramural biocompatibility and bioreactivity of such alloys and their components before and during degradation need to be assessed.
Abbreviations
QCA, quantitative coronary angiography
IVUS, intravascular ultrasound
REFERENCES
- 1.Colombo A, Karvouni E. Biodegradable stents “fulfilling the mission and stepping away”. Circulation 2000;102:371–3. [DOI] [PubMed] [Google Scholar]
- 2.Wintermantel E, Mayer J, Ruffieux K, et al. Biomaterialien: humane Toleranz und Integration. Chirurg 1999;70:847–57. [DOI] [PubMed] [Google Scholar]
- 3.Van der Giessen WJ, Lincoff AM, Schwartz RS, et al. Marked inflammatory sequelae to implantation of biodegradable and non-biodegradable polymers in porcine coronary arteries. Circulation 1996;94:1690–7. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RS, Edwards WD, Bailey KR, et al. Differential neointimal response to coronary artery injury in pigs and dogs. Implications for restenosis models. Arterioscler Thromb 1994;14:395–400. [DOI] [PubMed] [Google Scholar]
- 5.Farb A, Sangiorgi G, Carter AJ, et al. Pathology of acute and chronic coronary stenting in humans. Circulation 1999;99:44–52. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 1992;19:267–74. [DOI] [PubMed] [Google Scholar]
- 7.Lincoff AM, Furst JG, Ellis SG, et al. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J Am Coll Cardiol 1997;29:808–16. [DOI] [PubMed] [Google Scholar]
- 8.Tamai H, Igaki K, Kyo E, et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 2000;102:399–404. [DOI] [PubMed] [Google Scholar]
- 9.Peng T, Gibula P, Yao K-de, et al. Role of polymers in improving the results of stenting in coronary arteries. Biomaterials 1996;17:685–94. [DOI] [PubMed] [Google Scholar]
- 10.Cheng GC, Briggs WH, Gerson DS, et al. Mechanical strain tightly controls fibroblast growth factor-2 release from cultured human vascular smooth muscle cells. Circ Res 1997;80:28–36. [DOI] [PubMed] [Google Scholar]
- 11.Hofma SH, Whelan DMC, van Beusekom HMM, et al. Increasing arterial wall injury after long-term implantation of two types of stent in a porcine coronary model. Eur Heart J 1998;19:601–9. [DOI] [PubMed] [Google Scholar]
- 12.Sawyer PN, Brattain WH, Boddy PJ. Electrochemical criteria in the choice of materials used in vascular prosthesis. In: Sawyer PN, ed. Biophysical mechanism in vascular hemostasis and intravascular thrombosis. New York: Appleton-Century-Crofts 1965:337–48.
- 13.Underwood EJ. Trace elements in human and animal nutrition, 4th ed. New York: Academic Press, 1977.
- 14.Ferrando WA. Review of corrosion and corrosion control of magnesium alloys and composites. J Mater Eng 1989;11:4. [Google Scholar]
- 15.Nicole R. Metallschädigung bei Osteosynthesen. Helv Chir Acta 1947;14(suppl.III):5–70. [Google Scholar]
- 16.Haferkamp H, Kaese V, Niemeyer M, et al. Magnesium-base-alloys as implant-material; steps to the production of thin components. In: Aghion E, Eliezer D, ed. Proceedings of the 2nd Israeli International Conference on Magnesium Science and Technology 2000. Potash House, Israel: Magnesium Research Institute (MRI) Ltd, 2000:159–64.
- 17.Bauerschmidt P, Schaldach M. The electrochemical aspects of the thrombogenicity of a material. J Bioeng 1977;1:261–78. [Google Scholar]
- 18.Alt E, Haehnel I, Beilharz C, et al. Inhibition of neointima formation after experimental coronary artery stenting. A new biodegradable stent coating releasing hirudin and the prostacyclin analogue iloprost. Circulation 2000;10:1453–8. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz RS, Edwards WD, Bailey KR, et al. Differential neointimal response to coronary artery injury in pigs and dogs; implications for restenosis model. Arterioscler Thromb 1994;14:395–400. [DOI] [PubMed] [Google Scholar]
- 20.De Scheerder I, Wang K, Wilczek K, et al. Local methylprednisolone inhibition of foreign body response to coated intracoronary stents. Coron Artery Dis 1996;7:161–6. [DOI] [PubMed] [Google Scholar]
- 21.Lincoff AM, van der Giessen WJ, Sachwartz R, et al. Biodegradable and biostable polymers may both cause vigorous inflammatoty responses when implanted in the porcine artery. J Am Coll Cardiol 1993;21:179A. [Google Scholar]
- 22.Ungetüm M, Winkler-Gniewek W. Metallische Werkstoffe in der Orthopädie und Unfallchirurgie. Stuttgart: Georg Thieme Verlag, 1989.
- 23.Greaves I, Yates JR. The growth of freely initiating fatigue cracks in an unnnotched SiC/titanium composite. Int J Fatigue 1997;19:151–9. [Google Scholar]
- 24.Kirkpatrick CJ, Mittermayer C. Theoretical and practical aspects of testing potential biomaterials in vitro. J Mater Sci Mater Med 1990;1:9–13. [Google Scholar]
- 25.Komatsu R, Ueda M, Naruko T, et al. Neointimal tissue response at sites of coronary stenting in humans; macroscopic, histological, and immunhistological analyses. Circulation 1998;98:224–33. [DOI] [PubMed] [Google Scholar]
- 26.Farb A, Sangiori G, Carter AJ, et al. Pathology of acute and chronic coronary stenting in humans. Circulation 1999;99:44–52. [DOI] [PubMed] [Google Scholar]
- 27.Kornowski R, Hong MK, Fermin OT, et al. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol 1998;31:224–30. [DOI] [PubMed]