Abstract
Objective: To investigate whether enhanced oxidant stress in patients undergoing percutaneous transluminal coronary angioplasty (PTCA) is associated with a higher concentration of non-high density lipoprotein (HDL) cholesterol at baseline, and whether this contributes to the inflammatory reaction and luminal renarrowing after PTCA.
Design: An ex vivo and in vitro study of 46 patients who underwent PTCA and who had repeat angiograms after six months. Blood samples were collected immediately before PTCA, and at 24 hours, 48 hours, and 15 days after.
Setting: Tertiary referral centre.
Subjects: 46 patients (30 male, 16 female; mean (SD) age, 62 (5) years) with stable or unstable angina who underwent elective PTCA.
Main outcome measures: Continuous variable luminal loss as defined by change in minimum lumen diameter during follow up, normalised for vessel size; lag phase of low density lipoprotein to in vitro oxidation; plasma fluorescent products of lipid peroxidation (FPLP); plasma vitamin C and E; interleukin (IL) 1β secretion from unstimulated monocytes; plasma C reactive protein (CRP).
Results: Restenosis occurred in 12 patients (26%). Oxidant stress after PTCA was greater (p < 0.0001 at 15 days) in the patients with restenosis and showed a significant correlation with the preprocedural concentration of non-HDL cholesterol (p < 0.001). Inflammatory reaction (as reflected by IL-1β production and CRP) and late lumen loss were linearly correlated (p < 0.001) with lag phase and FPLP throughout the study, and inversely (p < 0.05) with vitamin C and E measured at two and 15 days after PTCA.
Conclusions: This study provides evidence for the critical role of cholesterol dependent oxidant stress in the pathophysiology of restenosis after PTCA. The findings raise the possibility that drugs capable of modulating oxidant status might provide a novel form of adjuvant treatment in patients with hypercholesterolaemia undergoing PTCA.
Keywords: cholesterol, oxidant stress, interleukin 1β, restenosis
Restenosis after percutaneous transluminal coronary angioplasty (PTCA) is still a major clinical problem.1 Recent studies have suggested a promising role for probucol, a lipid lowering agent with powerful antioxidant properties, in the prevention of restenosis.2 However, the underlying mechanisms of its benefit remain controversial.
Raised total cholesterol and low high density lipoprotein (HDL) cholesterol concentrations in serum are associated with coronary artery disease,3 and with increased production of reactive oxygen species (ROS).4 Notably, immunisation with homologous oxidised low density lipoprotein (LDL) reduces neointimal formation after balloon injury in hypercholesterolaemic rabbits.5 Furthermore, the newly discovered ACAT inhibitor F-1394 prevented intimal hyperplasia induced by balloon injury in rabbits.6 Finally, LDL apheresis, when applied before and after PTCA, is effective in preventing restenosis of coronary artery lesions in patients with coronary artery disease and hypercholesterolaemia.7
The generation of ROS and oxidation of lipids have profound and wide ranging effects that can dramatically increase vascular toxicity and initiate a cascade of molecular and cellular responses. In particular, there is now substantial evidence that oxidative modification of LDL may play an important role in restenosis by inducing endothelial dysfunction and enhancing the recruitment, intimal retention, and phenotypic transformation of monocytes.5 In addition, Liao and colleagues have recently proposed mechanisms whereby lipid peroxidation products—such as those generated during the oxidative modification of LDL—may induce expression of inflammatory mediator genes by activating nuclear factor (NF) κB-like transcription factors in vascular cells.8 In fact, oxidised LDL (oxLDL) may induce expression of adhesion molecules and cytokines such as interleukin (IL) 1β in circulating monocytes and arterial wall cells.9 It is assumed that IL-1β is an important determinant of intimal hyperplasia, amplifying and sustaining the proliferative response to vessel injury.10 Furthermore, it has been reported that plasma from ischaemic and reperfused hearts stimulates the expression of IL-1β in leucocytes from healthy donors.11
In humans, the association between hypercholesterolaemia and oxidative modifications of LDL has been well documented using both in vitro12 and in vivo10 markers of lipid peroxidation. Moreover, treatment with probucol has been reported to be effective in reducing plasma cholesterol and preventing LDL oxidation,13 IL-1β secretion,14 and smooth muscle cell growth.15 However, although atherosclerosis has long been associated with oxidation of LDL and lipoprotein,13,14,16 the relative importance of oxidative stress in mediating the proinflammatory effects of hypercholesterolaemia in the setting of coronary angioplasty is still unclear.
We speculated that enhanced oxidant stress in patients with higher concentrations of atherogenic cholesterol could induce the generation of inflammatory cytokines, and that these compounds might in turn contribute to restenosis after PTCA. In the present study, therefore, we investigated whether altered oxidant/antioxidant balance in patients undergoing PTCA is associated with the rate of luminal renarrowing after PTCA, and whether it correlates with a higher preprocedural concentration of non-HDL cholesterol and with the inflammatory reaction after PTCA.
METHODS
Patients
The study population consisted of 46 of 96 consecutive patients with stable or unstable angina pectoris who underwent elective PTCA on a single, non-occluded, stenosed coronary vessel. There were no differences between the patients with respect to any of the classic cardiovascular risk factors. Patients with factors that could influence the lipid profile (such as alcohol abuse, thyroid disease, kidney disease, diabetes, and so on) were excluded from the study. None of the participating subjects was taking vitamins, dietary supplements, or drugs with known antioxidant activity. All patients received aspirin (100 mg daily) for the entire study period. The baseline characteristics of the study subjects are given in table 1.
Table 1.
Baseline characteristics of the study patients
| Variable | No restenosis (n=34) | Restenosis (n=12) |
| Age (years) | 62 (5) | 62 (4.5) |
| Male/female | 22/12 | 8/4 |
| Stable angina/unstable angina | 11/23 | 4/8 |
| Total cholesterol (mmol/l) | 5.85 (0.31) | 7.10 (0.35) |
| HDL cholesterol (mmol/l) | 1.32 (0.23) | 1.14 (0.21) |
| Non-HDL cholesterol (mmol/l)* | 4.53 (0.26) | 5.96 (0.41) |
| Triglycerides (mmol/l) | 1.63 (0.49) | 1.71 (0.59) |
| Patients with: | ||
| Previous myocardial infarction | 0 | 0 |
| Previous PTCA or CABG | 0 | 0 |
| Family history of IHD | 14 | 5 |
| Hypertension | 22 | 8 |
| Diabetes mellitus | 0 | 0 |
| Smokers | 0 | 0 |
| Treatment with: | ||
| Calcium channel blockers | 18 | 6 |
| Nitrates | 26 | 9 |
| Unfractionated sodium heparin | 34 | 12 |
| Aspirin | 34 | 12 |
| Ticlopidine | 34 | 12 |
| Statins | 11 | 4 |
| Fibrates | 0 | 0 |
| Culprit vessel : | ||
| RCA | 12 | 4 |
| LAD | 14 | 5 |
| LCx | 8 | 3 |
| Balloon inflations | 5 | 5 |
| Maximum pressure (atm) | 12.3 (3.0) | 12.2 (4.0) |
| Total inflation time (s) | 498 (126) | 487 (117) |
| Stent implantation | 0 | 0 |
Values are mean (SD) or n. *p < 0.05.
CABG, coronary artery bypass grafting; HDL, high density lipoprotein; IHD, ischaemic heart disease; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; PTCA, percutaneous transluminal coronary angioplasty; RCA, right coronary artery.
Informed consent was obtained from each subject. The study protocol was approved by the institutional ethics committee.
Angioplasty procedure and follow up evaluation
Balloon angioplasty was undertaken using standard techniques. All patients received an intracoronary bolus of glyceryl trinitrate (1 mg) before the procedure. Patients were discharged with aspirin and any other necessary drugs. They were specifically asked not to take additional vitamins and mineral supplements and they were instructed in the American Heart Association step 1 diet. After successful angioplasty, patients returned for clinical follow up after 15 days and at one, three, and six months. They were readmitted for follow up coronary angiography six months after the angioplasty.
Quantitative coronary angiography
The four coronary arteriograms (obtained before the procedure, immediately afterwards, 15 minutes afterwards, and at the six months follow up visit) were analysed by an experienced cardiologist using the Philips H 4000 quantitative coronary analysis system (Philips Co, Einthoven, Netherlands). Measurements were made in a single projection showing the most severe stenosis. Whenever possible, the same projection was used in all four arteriograms to allow more accurate comparisons. The variation among repeated measurements of the percentage of stenosis was 7% when frames recorded one to six months apart were analysed.
Definitions
Restenosis in a previously successfully dilated lesion was defined as recurrent lumen diameter stenosis of more than 50% at follow up, as determined by quantitative coronary angiography.
The continuous variable luminal loss was defined as the change in minimum lumen diameter (MLD) during follow up normalised for vessel size, according to the following equation:
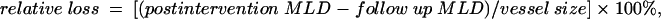 |
and reflects the degree of luminal renarrowing.17
Vessel size was the value of the reference diameter function at the minimal position of the obstruction.
Blood collection and biochemical measurements
Blood samples were collected from each patient immediately before PTCA and 24 hours, 48 hours, and 15 days afterwards. Blood was drawn into dark pyrogen-free test tubes containing EDTA (2.7 mM) as an anticoagulant and separated within one hour after sampling (2000 ×g for 20 minutes at 4°C). Immediately after separation, the plasma vitamin C concentration was measured spectrophotometrically at 595 nm, as described previously.18–20 In our laboratory, the intra-assay coefficient of variation for this method was 6.5%. In addition, total cholesterol concentration was determined by an enzymatic method, and HDL cholesterol was measured after phosphotungstic acid/MgCl2 precipitation of fresh plasma. Finally, aliquots of the plasma in EDTA were promptly stored at −80°C until LDL isolation and oxidisability analysis. Previous studies have shown that plasma storage and freezing/thawing does not affect LDL isolation or its major chemical characteristics.19
The LDL fraction was isolated by single vertical spin ultracentrifugation using a discontinuous NaCl/KBr density gradient, as previously reported.20 After dialysis against phosphate buffered saline (PBS), pH 7.4, at 4°C, LDL protein and cholesterol concentrations were determined by established methods.20 LDL oxidation (0.2 mg LDL cholesterol per millilitre) was triggered by the addition of 5 μM CuSO4 in PBS, pH 7.4, at 37°C. The lag phase preceding the formation of conjugated dienes and the LDL oxidisability as reflected by fluorescent products of lipid peroxidation (FPLP) was calculated as previously described.20 Finally, plasma vitamin E content was determined by high performance liquid chromatography.18
IL-1β release
The isolation and culture of peripheral blood monocytes from the study patients was done by an established method.17 The capacity of monocytes to produce IL-1β in vitro was determined as previously described by Pietersma and colleagues.17 Concentrations of IL-1β in supernatants were determined in triplicate by an enzyme immunoassay (Quantikine, R&D, Minneapolis, Minnesota, USA). The intra-assay and interassay coefficients of variation were less than 7%.
C reactive protein assay
C reactive protein (CRP) was assayed by an automated monoclonal antibody solid phase sandwich type enzyme immunoassay on an Abbott Imx instrument (Abbott Laboratories, Chicago, Illinois, USA), as previously described by Buffon and colleagues.21
Statistical analysis
For the clinical data, variables were compared using the χ2 test. An analysis of variance for repeated measures followed by a multiple comparison test (Sheffé test) was undertaken to test the changes in biochemical variables measured over time. Differences in biochemical variables between the restenotic and non-restenotic groups at each collection time were analysed by Student’s unpaired t test. The correlation between biochemical variables was assessed by the Spearman rank correlation test. The strength of the association of late lumen loss with each of the potential biological risk factors described in the previous sections was assessed by linear regression analysis.
Data are expressed as proportions or as mean (SD). Significance was assumed at a probability value of p < 0.05. All calculations were made using the SPSS 10.0.5 computer program.
RESULTS
Clinical course
No patient developed total acute obstruction at the angioplasty site immediately after the procedure or underwent urgent bypass surgery. None developed myocardial infarction and there were no sudden deaths during the study. There were no bleeding complications requiring blood transfusion. Restenosis occurred in 12 of the 46 patients (26%).
Comparison of oxidant status between patients with or without restenosis
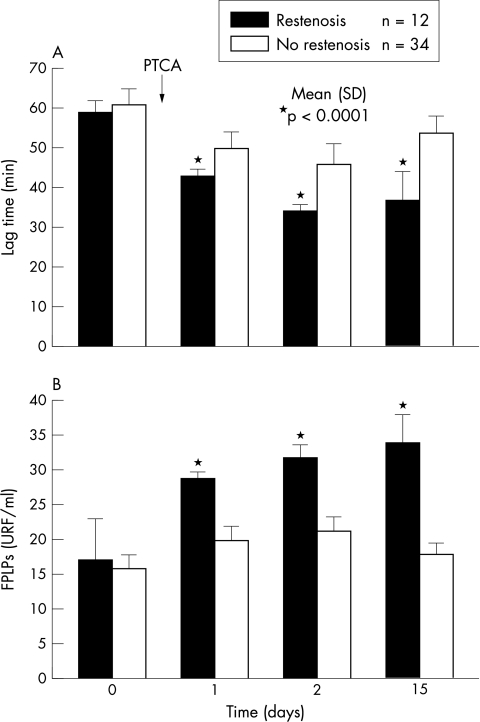
The susceptibility of LDL to in vitro oxidation was significantly increased after PTCA. The lag phase did not differ at baseline between the restenotic and non-restenotic groups (mean (SD), 59 (3) v 61 (4) minutes), but was significantly shorter (p < 0.0001) in the patients with restenosis at one day (43 (2) v 50 (4)), two days (34 (2) v 46 (5)), and 15 days (37 (7) v 54 (4)) after PTCA (fig 1). Likewise, plasma FPLP concentrations were significantly raised after the procedure (fig 1). There was no difference between the two groups before PTCA (17 (6) v 16 (2) URF (units of relative fluorescence)/ml); however, in the samples collected after the procedure there was a relatively stable increment in LDL oxidisability in the patients with restenosis and less consistent changes in those without restenosis. Overall, FPLP generation was significantly greater (p < 0.0001) in the patients with restenosis during the first (29 (1) v 20 (2)), second (32 (2) v 21 (2)), and 15th day (34 (4) v 18 (2)) of the study (fig 1).
Figure 1.
Time course of oxidant activity during the study period in the patients with or without restenosis. Low density lipoprotein (LDL) susceptibility to in vitro oxidation (A) and plasma fluorescent products of lipid peroxidation (FPLP) (B) were measured in 46 patients before (control) and at one, two, and 15 days after coronary angioplasty (PTCA). The bars and vertical lines represent mean and SD values.
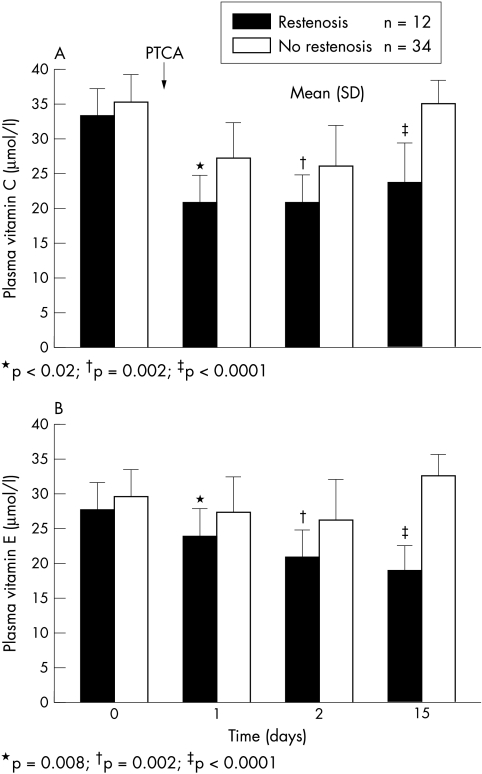
The time courses of the plasma antioxidant activities measured in the study are shown in fig 2. Antioxidant activity as reflected by vitamins C and E was significantly reduced after PTCA. Vitamin C did not differ at baseline (34 (4) v 36 (4) μmol/l), but was significantly lower in the patients with restenosis during the first (24 (4) v 28 (5) μmol/l; p < 0.02), second (21 (4) v 27 (2) μmol/l; p = 0.002), and 15th day (24 (5) v 36 (4) μmol/l; p < 0.0001) after PTCA. Similarly, vitamin E concentration was comparable in the two groups before PTCA (28 (4) v 30 (5) μmol/l), but declined significantly in the restenotic group at one day (24 (3) v 28 (4) μmol/l; p = 0.008), two days (21 (3) v 26 (4) μmol/l; p = 0.002), and 15 days (19 (3) v 33 (3) μmol/l; p < 0.0001) after the procedure.
Figure 2.
Time course of plasma antioxidant activity during the study period in the patients with and without restenosis. Plasma concentrations of vitamin C (A) and vitamin E (B) were measured in 46 patients before (control) and at one, two, and 15 days after coronary angioplasty (PTCA). The bars and vertical lines represent mean and SD values.
Relation between non-HDL cholesterol and oxidant stress
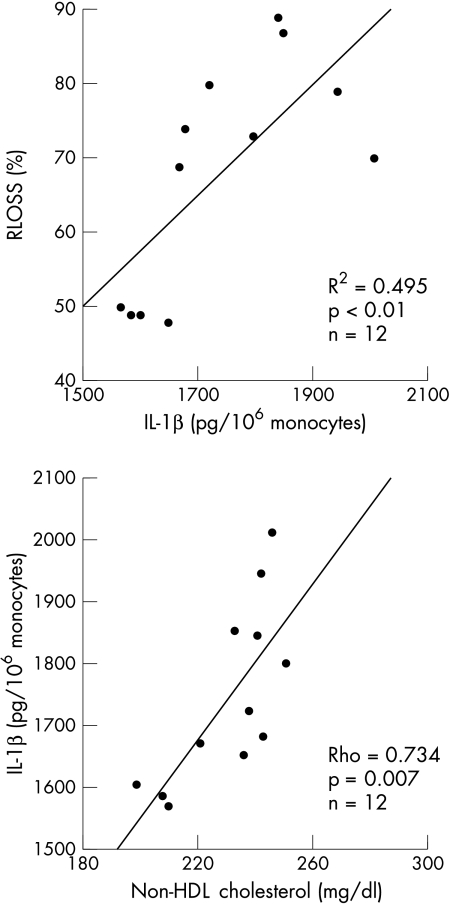
To analyse whether preprocedural concentration of atherogenic cholesterol can influence oxidative response after PTCA, we studied the correlation between non-HDL cholesterol measured at baseline and oxidant stress measured after the procedure. In the samples collected one, two, and 15 days after PTCA, we found correlations between the concentration of preprocedural non-HDL cholesterol and the following variables: lag phase (ρ = −0.574, p < 0.0001; ρ = −0.471, p = 0.001; and ρ = −0.622, p < 0.0001, respectively); FPLP (ρ = 0.460, p = 0.001; ρ = 0.488, p = 0.001; and ρ = 0.481, p = 0.001); vitamin C (ρ = −0.247, NS; ρ = −0.327, p < 0.003; and ρ = −0.584, p < 0.0001); and vitamin E (ρ = −0.252, NS; ρ = −0.427, p = 0.003; and ρ = −0.681, p < 0.0001).
Variables predictive of late lumen loss
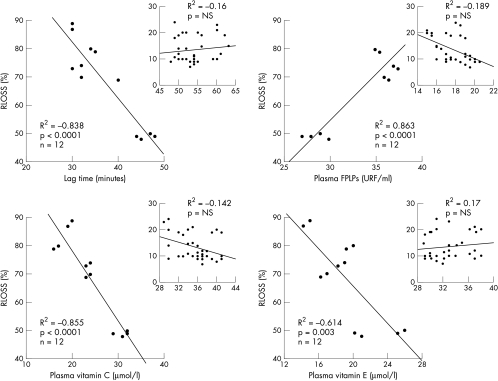
To investigate whether redox state could contribute to luminal renarrowing after PTCA, we assessed the association between oxidative burden determined at one, two, and 15 days after PTCA and the degree of luminal renarrowing (relative loss, RLOSS) at six months after angioplasty. We identified several univariate predictors of late lumen loss. Lag phase showed a significant inverse correlation with RLOSS in the samples collected at one day (R2 = −0.364, p < 0.0001), two days (R2 = −0.24, p = 0.001), and 15 days (R2 = −0.916, p < 0.0001) after PTCA. Similarly, a strong correlation (p < 0.0001) was found between plasma FPLP and RLOSS during the first day (R2 = 0.68, p < 0.0001), second day (R2 = 0.756), and 15th day (R2 = 0.898, p < 0.0001) after revascularisation. There was no significant correlation between plasma vitamin C and RLOSS on the first day after PTCA; however, an increasing inverse association was found on the second day (R2 = −0.319, p < 0.002) and on the 15th day (R2 = −0.806, p < 0.0001) of the study. Likewise, the inverse association between plasma vitamin E and RLOSS increased through the first (R2 = −0.123, p < 0.02), second (R2 = −0.381, p < 0.002), and 15th day (R2 = −0.787, p < 0.0001) after PTCA. The strong association between redox state and RLOSS was conserved when separate analyses were performed for subjects with restenosis (fig 3, panels A–C).
Figure 3.
Scatterplots showing association between lag time of in vitro LDL oxidation, plasma fluorescent products of lipid peroxidation (FPLP), plasma vitamin C, and plasma vitamin E measured in patients with and without restenosis (box) 15 days after coronary angioplasty and the degree of luminal renarrowing expressed as relative loss (RLOSS) at six months after treatment.
Monocyte release of IL-1β and plasma CRP
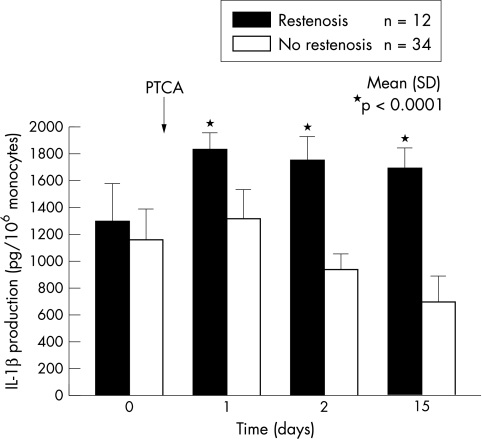
IL-1β secretion by unstimulated blood monocytes was particularly enhanced after PTCA in the patients with restenosis. The time course of IL-1β biosynthesis during the study is shown in fig 4. There was no notable difference between the two groups of patients before PTCA (1286 (283) v 1147 (234) pg/106 monocytes). However, in the successive samples collected after the procedure, IL-1β production was greater (p < 0.0001) in the patients with restenosis on the first (1827 (131) v 1306 (226) pg/106 monocytes), second (1741 (186) v 935 (112) pg/106 monocytes), and 15th day (1689 (149) v 694 (195) pg/106 monocytes). Similar differences were observed with respect to plasma CRP in the samples collected on the first day (13.4 (5.0) v 4.2 (1.0) mg/dl) and the second day (6.1 (1.4) v 2.2 (0.9) mg/dl) after PTCA. Moreover, IL-1β biosynthesis after PTCA showed a different trend in the two groups. In the patients with restenosis, enhanced IL-1β production remained significant throughout the study (p < 0.0001). Otherwise, in the patients without restenosis IL-1β release was greater 24 hours after PTCA (p = 0.006), but decreased spontaneously as a function of time, as reflected by the significant difference between the first and the 15th day after the procedure (1306 (226) v 694 (195) pg/106 monocytes, p < 0.0001). Finally, circulating IL-1β showed a significant correlation with RLOSS (p < 0.0001) during all the entire study period (fig 5A).
Figure 4.
Time course of the production of interleukin-1β (IL-1β) by unstimulated blood monocytes obtained from patients with or without restenosis before (control), and one, two, and 15 days after coronary angioplasty (PTCA). The bars and vertical lines represent mean and SD values.
Figure 5.
Correlations of interleukin-1β (IL-1β), measured 15 days after PTCA, with the degree of luminal renarrowing expressed as relative loss (RLOSS) at six months after treatment (upper panel), and with preprocedural non-HDL cholesterol (lower panel) in the 12 patients with restenosis.
Relation between oxidant stress and IL-1β production
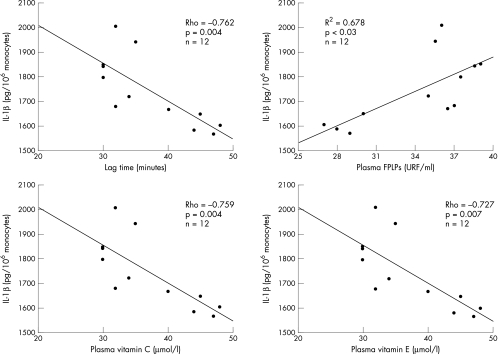
Finally, to investigate whether the cholesterol dependent redox state could influence restenosis by inducing an inflammatory reaction after PTCA, we analysed the correlation between non-HDL cholesterol, oxidant stress, and IL-1β production in the group of patients who developed late restenosis. In the samples collected one, two, and 15 days after PTCA, we found a significant association between IL-1β and the following variables: non-HDL cholesterol (ρ = 0.641, p < 0.01; ρ = 0.62, p < 0.03; and ρ = 0.734, p = 0.007, fig 5, respectively); lag phase (ρ = −0.741, p < 0.005; ρ = −0.563, p < 0.01; and ρ = −0.762, p = 0.004); FPLP (ρ = 0.714, p < 0.01; ρ = 0.59, p < 0.05; and ρ = 0.678, p < 0.03); vitamin C (ρ = −0.367, p < 0.05; ρ = −0.459, p < 0.05; and ρ = −0.759, p = 0.004); and vitamin E (ρ = −0.393, p < 0.03; ρ = −0.462, p < 0.03; and ρ = −0.727, p = 0.007) (fig 6).
Figure 6.
Correlation of lag time of in vitro LDL oxidation, plasma fluorescent products of lipid peroxidation (FPLPs), plasma vitamin C, and plasma vitamin E with the production of interleukin-1β (IL-1β) by unstimulated blood monocytes obtained from the 12 patients with restenosis 15 days after coronary angioplasty.
DISCUSSION
In this study we tested the hypothesis that a component of the restenosis process following coronary angioplasty is dependent on the enhanced and long lasting oxidant stress that is related to baseline non-HDL cholesterol concentration, and would have the capacity to regulate inflammatory reactions and vascular smooth muscle cell proliferation after reperfusion.
Although Nunes and colleagues demonstrated persistently increased O2− generation in injured vessels after experimental PTCA in animals,22 studies in humans have shown only transient increases in LDL oxidisability after PTCA.23,24 In fact, these studies have analysed only the period (a matter of minutes) immediately following myocardial reperfusion, and they have not compared oxidant status in subsets of patients with different outcomes after PTCA. Thus an original finding of the present study was that patients undergoing PTCA experienced a persistent increase in systemic oxidative stress, and this was significantly more obvious and prolonged in those with restenosis. Concurrent drug treatments are unlikely to have contributed to such different levels of lipid peroxidation as the treatment was similar in the two groups. Oxidant stress increased significantly in both groups 24 hours after PTCA; however, while it returned to baseline at 15 days in the patients without restenosis, in the patients with restenosis the changes persisted throughout the study. Thus the differences in oxidative burden between the two groups became apparent 24 hours after PTCA and remained significant until the final day of the study.
Our second finding was that enhanced oxidant stress after PTCA was related to the preprocedural concentration of non-HDL cholesterol, as suggested by the significant correlation between the two. Because oxidant stress has biological effects on vascular and monocyte function,4 enhanced production of oxidant compounds might mediate some of the effects of hypercholesterolaemia on important determinants of vascular occlusion, as suggested by the striking correlation between redox state and late lumen loss. Thus the ability of cholesterol dependent oxidant stress to induce and amplify monocyte activation may be relevant in settings where inflammation and enhanced lipid peroxidation coincide, such as PTCA.
Our final original finding was that the activation status of monocytes after PTCA—as reflected by spontaneous IL-1β release—was persistently higher in the group of patients who developed restenosis. That an increased oxidative burden after PTCA might have an effect on cytokine biosynthesis in influencing vascular healing was also suggested by the significant correlation between the circulating levels of indices of oxidative burden and the rate of IL-1β biosynthesis.
At least two alternative explanations could be considered for the mechanisms underlying the more impressive oxidant stress and monocyte activation following PTCA in the patients with restenosis. First, the diversity may simply have reflected differences in the degree of ischaemia/reperfusion during PTCA. However, this seems unlikely for the following reasons: all the procedures were done by the same experienced cardiologist according to standard techniques; the two groups had similar clinical and angiographic profiles at baseline; there were no significant differences in the total period of vessel occlusion during the procedure (table 1); and the differences in oxidant status between the two groups remained significant in the samples collected 15 days after PTCA. Alternatively, our data are consistent with the hypothesis that the high interindividual variability in the oxidative burden after revascularisation—which was critically related to preprocedural concentrations of non-HDL cholesterol—might, by governing early inflammatory events, contribute at least in part to the differences observed in the processes of healing, remodelling, and late lumen loss after PTCA in the presence of similar angiographic profiles.
In this study, a pronounced and long lasting systemic oxidant burden in the patients with restenosis was always characterised by a concomitant increase in oxidants and a decrease in antioxidant substances. We speculate that the reduction in vitamins C and E after PTCA in this group of patients may have reflected consumption resulting from interference of these vitamins with the pathways responsible for superoxide anion (O2−) production or perhydroxyl radical formation.24
The striking effect of PTCA in increasing lipid peroxidation raises the question of the origin of this oxidant burden. Macrophages and smooth muscle cells, the major types of cell left in the atherosclerotic lesion after PTCA, can both elaborate active oxygen species. Furthermore, granulocytes can also produce large amounts of O2− and other oxidants. Interestingly, it has been reported that inflammatory cells may accumulate after PTCA at sites of acute tissue trauma and thrombosis, not only in the injured intima but also in the adventitial layer surrounding the artery, which is increasingly recognised as a site of scarring and luminal constriction in restenotic lesions.25
There is increasing evidence to suggest that oxygen species might contribute to restenosis, including smooth muscle cell migration, replication, and accumulation and remodelling of the extracellular matrix. In particular, many vascular biologists now ascribe a central role to augmented transcription of several atherosclerosis related genes by the oxidant sensitive regulatory pathway involving NF-κB.26 Exposure to oxidant stress activates the NF-κB regulatory complex in vascular cells and triggers the transcription of genes that encode certain leucocyte adhesion molecules, chemoattractant cytokines, and enzymes that can influence extracellular matrix metabolism.27 Moreover, it has been proposed10 that the early acute cytokine generation in macrophages, which are a major source of IL-1β, could evoke a secondary cytokine and growth factor response from other types of cell in the lesion, including smooth muscle cells and endothelial cells. This would establish a positive, self stimulatory autocrine and paracrine feedback loop, amplifying and sustaining the proliferative response. The positive results of probucol (a lipid lowering drug with powerful antioxidant action) in preventing renarrowing after PTCA2 suggest that this agent might interrupt these or other oxidant sensitive signalling systems and thus mitigate the sustained proinflammatory state that follows balloon injury to arteries28 and ultimately reduce the restenosis rate. In contrast, the negative results observed with vitamin C and E in the same study,2 and with statins in several clinical trials,29 suggest that separate antioxidant or lipid lowering strategies are not sufficient to suppress the strong inflammatory reaction following PTCA, and that a synergistic lipid lowering and antioxidant approach is necessary to modulate this phenomenon.
Despite their originality, the results of our study must be viewed with caution because of the small sample size; thus a further study with a larger sample size will be necessary to confirm the data.
Conclusions
We believe that our findings have implications for both research and clinical practice. They have potential importance from a fundamental standpoint because they demonstrate the critical role of cholesterol dependent oxidant stress in the pathophysiology of restenosis after PTCA. From a practical standpoint, the findings raise the possibility that drugs capable of modulating oxidant status might provide a novel form of adjuvant treatment in patients with hypercholesterolaemia who undergo PTCA.
Abbreviations
CRP, C reactive protein
FPLP, fluorescent products of lipid peroxidation
HDL, high density lipoprotein
IL, interleukin
LDL, low density lipoprotein
PTCA, percutaneous transluminal coronary angioplasty
RLOSS, relative loss
ROS, reactive oxygen species
REFERENCES
- 1.Landau C, Lange RA, Hillis LD. Percutaneous transluminal coronary angioplasty. N Engl J Med 1994;330:981–93. [DOI] [PubMed] [Google Scholar]
- 2.Tardif JC, Côté G, Lespérance J, et al. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. N Engl J Med 1997;337:365–72. [DOI] [PubMed] [Google Scholar]
- 3.Solymoss BC, Marcil M, Wesolowska E, et al. Relation of coronary artery disease in women <60 years of age to the combined elevation of serum lipoprotein (a) and total cholesterol to high-density cholesterol ratio. Am J Cardiol 1993;72:1215–19. [DOI] [PubMed] [Google Scholar]
- 4.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 1993;91:2546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson J, Calara F, Regnstrom J, et al. Immunization with homologous oxidized low density lipoprotein reduces neointimal formation after balloon injury in hypercholesterolemic rabbits. Am Coll Cardiol 1997;30:1886–91. [DOI] [PubMed] [Google Scholar]
- 6.Aragane K, Fujinami K, Kojima K, et al. ACAT inhibitor F-1394 prevents intimal hyperplasia induced by balloon injury in rabbits. J Lipid Res 2001;42:480–8. [PubMed] [Google Scholar]
- 7.Adachi H, Niwa A, Shinoda T. Prevention of restenosis after coronary angioplasty with low-density lipoprotein apheresis. Artif Organs 1995;19:1243–7. [DOI] [PubMed] [Google Scholar]
- 8.Liao F, Andalibi A, Qiao JH, et al. Genetic evidence for a common pathway mediating oxidative stress, inflammatory gene induction, and aortic fatty streak formation in mice. J Clin Invest 1994;94:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn MT, Parthasarathy S, Fong LG, et al. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA 1987;84:2995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P, Schwartz D, Brogi E, et al. A cascade model for restenosis: a special case of atherosclerosis progression. Circulation 1992;86(suppl III):III-47–52. [PubMed] [Google Scholar]
- 11.Marx N, Neumann FJ, Ott I, et al. Induction of cytokine expression in leukocytes in acute myocardial infarction. J Am Coll Cardiol 1997,30:165–70. [DOI] [PubMed] [Google Scholar]
- 12.Thomas CE, Jackson RL, Ohlweiler DF, et al. Multiple lipid products in low density lipoproteins induce interleukin-1 beta release from human blood mononuclear cells. J Lipid Res 1994;35:417–27. [PubMed] [Google Scholar]
- 13.Parthasarathy S, Young SG, Witztum JL, et al. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest 1986;77:641–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ku G, Doherty NS, Wolos JA, et al. Inhibition by probucol of interleukin 1 secretion and its implication in atherosclerosis. Am J Cardiol 1988;62:77–81B. [DOI] [PubMed] [Google Scholar]
- 15.Schneider JE, Berk BC, Gravanis MB, et al. Probucol decreases neointimal formation in a swine model of coronary artery balloon injury: a possible role for antioxidant in restenosis. Circulation 1993;88:628–37. [DOI] [PubMed] [Google Scholar]
- 16.Daví G, Alessandrini P, Mezzetti A, et al. In vivo formation of 8-epi-prostaglandin F2α is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol 1997;17:3230–5. [DOI] [PubMed] [Google Scholar]
- 17.Pietersma A, Kofflard M, De Wit EA, et al. Late lumen loss after coronary angioplasty is associated with the activation status of circulating phagocytes before treatment. Circulation 1995;91:1320–5. [DOI] [PubMed] [Google Scholar]
- 18.Day BR, Williams DR, Marsh CA. A rapid manual method for routine assay of ascorbic acid in serum and plasma. Clin Biochem 1979;12:22–6. [DOI] [PubMed] [Google Scholar]
- 19.Mezzetti A, Lapenna D, Pierdomenico SD, et al. Vitamins E, C and lipid peroxidation in plasma and arterial tissue of smokers and non-smokers. Atherosclerosis 1995;112:91–9. [DOI] [PubMed] [Google Scholar]
- 20.Mezzetti A, Guglielmi MD, Pierdomenico SD, et al. Increased systemic oxidative stress after elective endarterectomy: relation to vascular healing and remodeling. Arterioscler Thromb Vasc Biol 1999;19:2659–65. [DOI] [PubMed] [Google Scholar]
- 21.Buffon A, Liuzzo G, Biasucci LM, et al. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol 1999;34:1512–21. [DOI] [PubMed] [Google Scholar]
- 22.Nunes GL, Robinson K, Kalynych A, et al. Vitamins C and E inhibit O2− production in the pig coronary artery. Circulation 1997;96:3593–601. [DOI] [PubMed] [Google Scholar]
- 23.Cipollone F, Marini M, Fazia M, et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol 2001;21:327–34. [DOI] [PubMed] [Google Scholar]
- 24.Mezzetti A, Lapenna D, Pierdomenico SD, et al. Vitamins E, C, and lipid peroxidation in plasma and arterial tissue of smokers and non-smokers. Atherosclerosis 1995;112:91–9. [DOI] [PubMed] [Google Scholar]
- 25.Scott NA, Cipolla GD, Ross CE, et al. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation 1996;93:2178–87. [DOI] [PubMed] [Google Scholar]
- 26.Collins T, Read MA, Neish AS, et al. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J 1995;9:899–909. [PubMed] [Google Scholar]
- 27.Mezzetti A, Cipollone F, Cuccurullo F. Oxidative stress and cardiovascular complications in diabetes: isoprostanes as new markers on an old paradigm. Cardiovasc Res 2000;47:475–88. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H, Sukhova GK, Swanson SJ, et al. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation 1993;88:1788–803. [DOI] [PubMed] [Google Scholar]
- 29.Serruys PW, Foley DP, Jackson G, et al. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J 1999;20:58–69. [DOI] [PubMed] [Google Scholar]