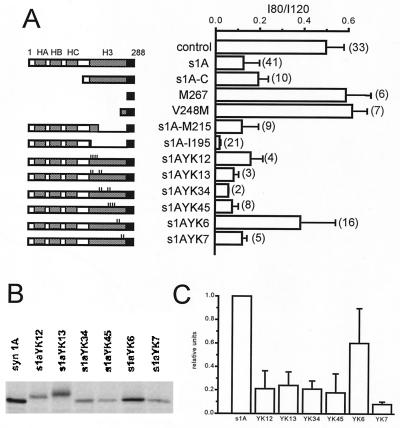
Figure 3.
The helical H3 domain of syntaxin 1A contains structural determinants critical for the modulatory interaction with N-type Ca2+ channels. (A) Bar graph displays mean ± SEM values of the I80/I120 ratio for each mutant construct (number of oocytes shown in parentheses). The mutant constructs are described in Materials and Methods. Domain deletions in s1A-C, M267, V248 M, s1A-M215, and s1A-I195 are as indicated. The small vertical lines denote point mutations in s1A-H3 point mutants. Of the various point mutant constructs, only s1A-YK6 shows an I80/I120 ratio significantly greater than wild-type s1A. For further details, see text. (B) In vivo 35S-Met labeling of s1A constructs expressed in Xenopus oocytes and immunoprecipitation with HPC-1 antibody. (C) Levels of 35S-Met labeling, normalized by expression level of wild-type syntaxin 1A in each experiment, shown as mean ± SEM.