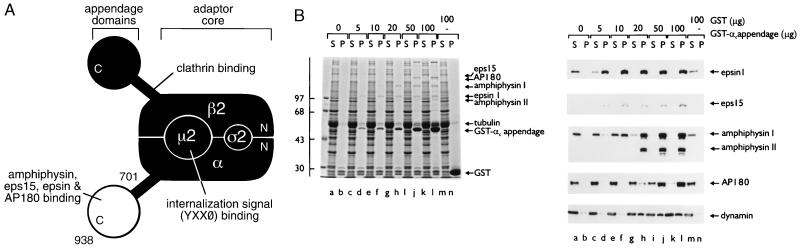
Figure 1.
(A) Schematic illustration of the subunit organization of the AP-2 adaptor heterotetramer. Regions of the complex with known protein-binding functions are indicated. For tyrosine-based internalization signals, X is any amino acid and Ø represents a bulky hydrophobic residue (1, 3). (B) Functional protein associations with the GST-αC-appendage fusion protein. Purified GST-αC appendage (0–100 μg) or GST (100 μg), immobilized on 25 μl packed glutathione Sepharose, were incubated in ≈7.5 mg/ml rat brain cytosol for 60 min at 4°C. The Sepharose beads were then recovered by centrifugation, and aliquots corresponding to ≈1/150 of the supernatant (S) and 1/10 of each pellet (P) were resolved by SDS/PAGE and either stained with Coomassie blue (Left) or transferred to nitrocellulose (Right). Portions of the blots were probed with anti-epsin, anti-eps15, anti-amphiphysin, anti-AP180, or anti-dynamin antibodies. The position of the markers (kDa) is indicated on the left and only the relevant portion of each blot is shown.