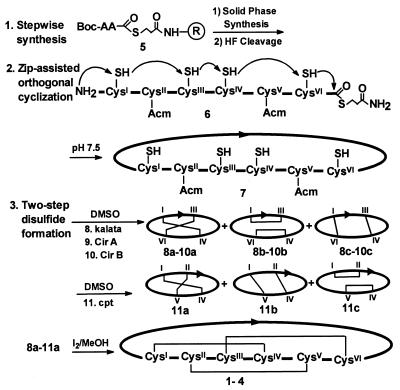
Figure 2.
Synthetic scheme for preparing the cystine-knot macrocycles and their analogs. Linear precursors were prepared by stepwise solid-phase synthesis. The freed peptides 6 without side chain protecting groups were cyclized in aqueous solutions at pH 7.5 through the thia zip reaction to afford macrocycles 7. The SS-protection schemes for kalata, CirA, and CirB were Cys II-V (Acm) to give the two-SS isomers 8–10 and Cys III-VI for Cpt to yield 11 after oxidation in 15% DMSO in aqueous buffer. Oxidation by I2 resulted in macrocycles 1–4.