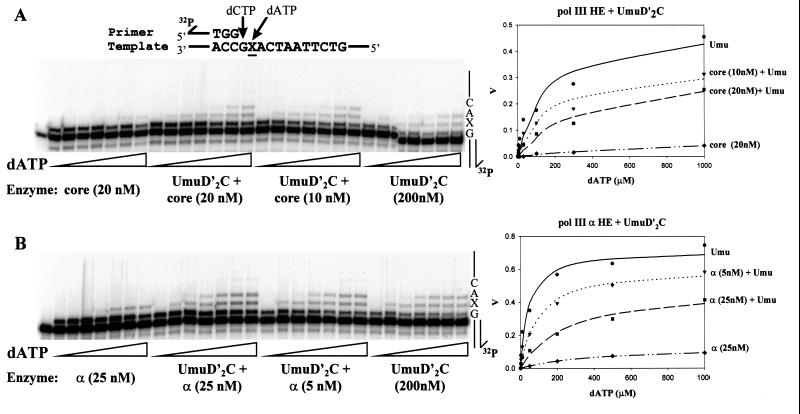
Figure 4.
Inhibition of UmuD′2C (E. coli pol V)-catalyzed translesion synthesis by wild-type pol III. A running-start C (20 μM dCTP) is incorporated opposite template G before reaching the abasic lesion, X. The concentration of dATP used for incorporation opposite X was varied to measure the kinetics of incorporation by either UmuD′2C, wild-type pol III, or a combination of both proteins. The UmuD′2C complex used in the reactions was purified from a strain containing wild-type pol III (18). Michaelis–Menten saturation plots of the translesion synthesis rate vs. dATP concentration are shown at the right. The translesion synthesis rate V is obtained by measuring IXΣ/IX−1 as a function of dATP concentration, where IXΣ are the integrated gel band intensities for incorporation at the site of the lesion and beyond, and IX−1 is the integrated gel band intensity at the G site, before reaching the lesion (see Materials and Methods). (A) Translesion synthesis catalyzed by UmuD′2C, pol III core HE, or combinations of both. (B) Translesion synthesis catalyzed by UmuD′2C, pol III α HE, or combinations of both. The dATP concentrations used were 0, 3, 10, 30, 100, 300, and 1,000 μM for A and 0, 2, 10, 50, 200, 500, and 1,000 μM for B. UmuD′2C is present at 200 nM in each experiment. The reactions were run at 37°C in the presence of RecA, SSB, and β, γ complex.