Abstract
Aims: The presence of granulomas within the stroma of carcinomas and in the lymph nodes draining carcinomas has been well described. To date, however, there have been few studies examining the occurrence and relevance of necrobiotic granulomas occurring in association with breast carcinoma.
Methods/Results: Four cases of breast carcinoma with necrobiotic granulomas were examined using periodic acid Schiff and Ziehl Neelsen stains for fungi and tubercle bacilli and with immunohistochemistry using CAM5.2, cytokeratin 7, and cytokeratin AE1/3 for tumour cells. In one case the stroma was involved, in the other three cases the lymph nodes contained necrobiotic granulomas. In two of the cases, one with stromal and one with lymph node involvement, the necrobiotic granulomas contained necrotic tumour cells.
Conclusion: In this study the features of four cases of breast carcinomas with necrobiotic granulomas are examined and their relevance explored. Close scrutiny of such granulomas is necessary to avoid underdiagnosis of metastatic disease.
Keywords: necrobiotic palisading granuloma, breast carcinoma, metastases, cytokeratins
Non-caseating epithelioid granulomas can occur in the lymph nodes draining carcinomas and less commonly within the stroma of carcinomas.1,2 These are considered to be the result of a type IV autoimmune hypersensitivity reaction to tumour antigens or non-specific variants of common carcinomas. Necrobiotic palisading granulomas are not often associated with breast carcinoma, and their presence may provoke a greater consideration of additional differential diagnostic considerations of granulomatous inflammation. This report describes four such cases, one involving the tumour stroma and three the draining lymph nodes.
CASE REPORTS
Case 1
A 55 year old woman presented with breast carcinoma measuring 5.0 cm. Microscopic examination showed a grade 2 infiltrating ductal carcinoma with one of the 10 lymph nodes containing a small necrotising granuloma (fig 1). This was composed of central amorphous, eosinophilic, fibrinoid-like, necrotic material surrounded by fibrosis and palisaded histiocytes. Ziehl Neelsen (ZN) and periodic acid Schiff (PAS) stains were negative, as was cytokeratin (CK) immunostaining (AE1/3, Cam5.2). The lesion cut out before epithelial membrane antigen (EMA) and CK7 immunostaining was performed. There was no other relevant history. The patient is well five months after surgery.
Figure 1.
Necrotising granuloma in lymph node surrounded by fibrosis and palisaded histiocytes (case 1).
Case 2
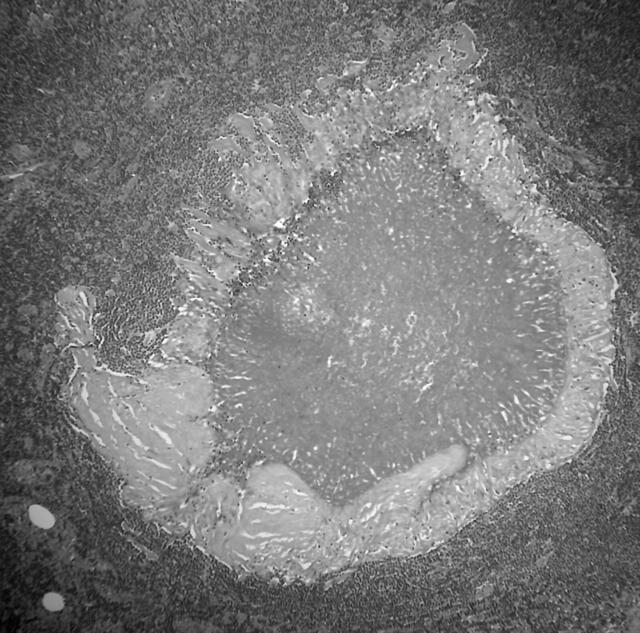
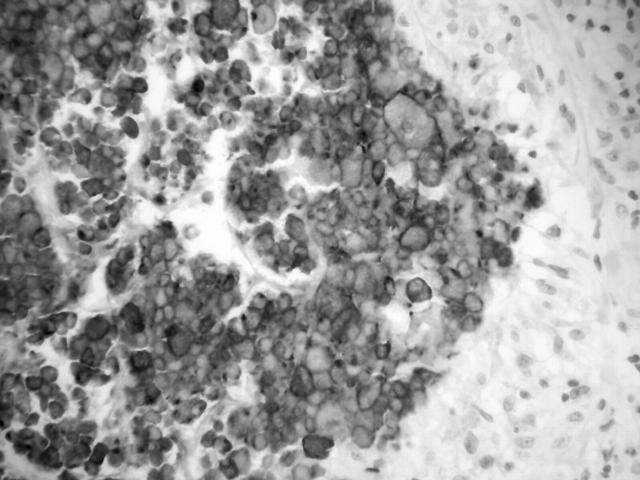
In 1976, a 50 year old woman had a left mastectomy for infiltrating carcinoma with a recurrence in 1981 in the left axilla. She subsequently underwent radiotherapy and a course of tamoxifen for two years. At the age of 74 she presented with a mass in the right breast and enlarged axillary lymph nodes. A right mastectomy was performed and microscopic examination revealed a grade 3 invasive ductal carcinoma. A few small poorly formed epithelioid granulomas were present. Fourteen of 20 lymph nodes contained metastatic carcinoma and nine of the 20 nodes contained both epithelioid, non-caseating granulomas and palisaded necrotising granulomas. Some nodes contained poorly formed epithelioid granulomas, a few contained metastatic carcinoma and epithelioid granulomas, and two contained palisaded necrotising granulomas, one also with occasional small epithelioid granulomata. These necrotising granulomas showed eosinophilic, amorphous fibrinoid-like necrotic centres surrounded by palisaded histiocytes and occasional Langhan-type giant cells (fig 2). ZN and PAS stains were negative. CK7 and AE1/3 immunohistochemistry showed large numbers of strongly positive, circumferentially membrane stained tumour cells within the necrotic centres (fig 3). Cam5.2 and EMA showed focal positivity, and a few residual tumour nuclei with nucleoli were visible on the haematoxylin and eosin section on close scrutiny. A single epithelioid granuloma contained a CK positive tumour cell, whereas the remainder were located immediately adjacent to or distant from associated metastatic carcinoma. The patient received radiotherapy and tamoxifen. She re-presented 17 months later with increasing dyspnoea, unstable angina, and melaena. A computed tomography scan of the abdomen revealed multiple enlarged abdominal lymph nodes and plaques of tumour on the duodenum and caecum, thought to be consistent with metastatic breast carcinoma. There was also evidence of pulmonary metastases and she died a week later.
Figure 2.
Necrotising granuloma in lymph node with histiocytes and occasional giant cells (case 2).
Figure 3.
Cytokeratin AE1/3 membrane reactivity in necrotic centre outlining residual tumour cells (case 2).
Case 3
A 79 year old woman presented with a 20 × 13 cm, fungating tumour fixed to the right chest wall and a left breast tumour measuring 5.0 × 7.0 cm, which was also fungating, but was not attached to the underlying tissue. She was given six cycles of chemotherapy (cyclophosphamide, methotrexate, and fluorouracil) to which she had a good response with shrinkage of both tumours. She was given tamoxifen for just under four months, at which time she underwent bilateral mastectomy. The right mastectomy specimen contained a 7.0 cm carcinoma reaching the deep margin and the left mastectomy specimen contained a fungating tumour measuring 6.0 cm in maximum dimension. Microscopic examination showed the right breast carcinoma to be a grade 3 infiltrating lobular carcinoma of mixed type with foci of lobular carcinoma in situ. Oestrogen and progesterone receptors were negative. Close to the deep margin and adjacent to the carcinoma there were two necrobiotic granulomas with central amorphous eosinophilic fibrinoid-like necrosis surrounded by palisaded histiocytes and Langhan-type giant cells (fig 4). PAS and ZN stains were negative. Immunohistochemistry for CKs (CAM5.2, AE1/3) and EMA showed frequent circumferentially membrane stained cells within the necrotic centre, with staining for CK7 being the strongest and seen in the largest number of cells. On very close inspection of the haematoxylin and eosin stained section, a very few tumour nuclei with prominent nucleoli were visible. The carcinoma extended to within 5 mm of the deep excision margin. Twelve lymph nodes showed no evidence of metastatic carcinoma. Microscopic examination of the left breast tumour showed an infiltrating ductal carcinoma, grade 3. This extended to within 3 mm of the deep excision margin and four of 12 lymph nodes contained metastatic carcinoma. Oestrogen receptors were weakly positive in most nuclei and progesterone receptors were strongly positive in approximately 10% of nuclei. The patient is well with no clinical evidence of recurrence three years later.
Figure 4.
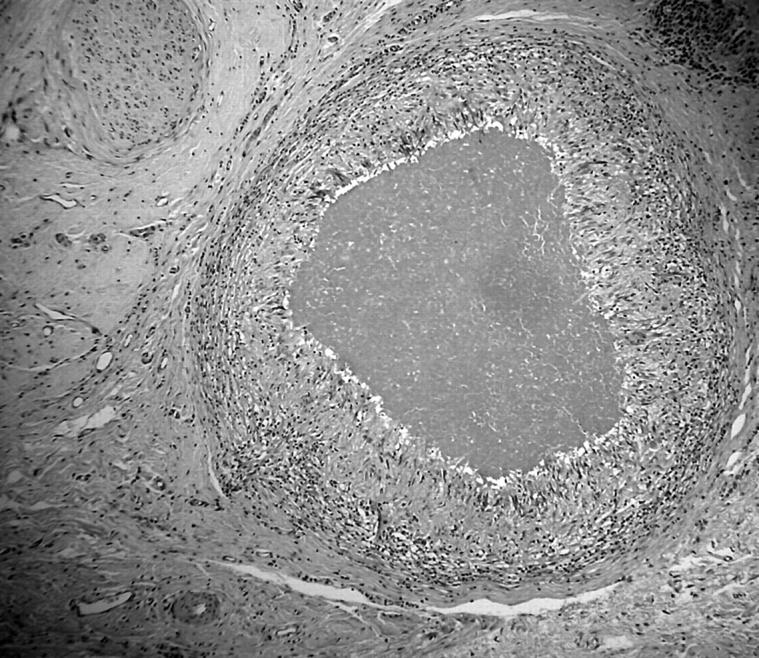
Stromal necrotising granuloma comprising palisaded histiocytes and giant cells (case 3).
Case 4
A 58 year old woman was found on screening mammogram to have a radiologically malignant lesion in the left breast. Fine needle aspiration showed malignant cells and a wide local excision with axillary node clearance was performed. Microscopic examination showed an infiltrating ductal carcinoma grade 2, measuring 15 mm in maximum dimension, and there was no evidence of tumour necrosis. Stromal granulomas were not present. However, two of nine lymph nodes contained necrotising, palisaded granulomas composed of amorphous, eosinophilic, fibrinoid-like necrotic centres surrounded by fibrosis, palisaded histiocytes, and occasional Langhan-type giant cells, one of which was noted macroscopically as a milky white area. Three additional lymph nodes contained small epithelioid granulomas. ZN and PAS stains were negative, as was CK (CAM5.2, AE1/3, CK7) and EMA immunohistochemistry. There was no other relevant history. She received postoperative radiotherapy and was on tamoxifen for five years. The patient is well seven years after wide local excision.
DISCUSSION
The presence of sarcoid-type granulomas in lymph nodes draining carcinomas, including breast carcinomas, was first reported in detail by Gorton and Linell.1 In the specific context of breast carcinoma, Oberman reported three cases of epithelioid granulomas within the stroma adjacent to invasive carcinomas of the breast, but without involvement of axillary nodes.2 Alujevic et al and Santini et al each separately reported a case of invasive breast carcinoma with a granulomatous response and deposition of stromal amyloid.3,4 Daroca described three cases of medullary carcinoma of the breast with stromal granulomas.5 Bässler and Birke reported five cases of breast carcinoma with stromal granulomas; four of these were infiltrating ductal carcinomas and one was an infiltrating lobular carcinoma.6 One case (their case three) had a single necrobiotic stromal granuloma in the primary breast carcinoma and the recurrent carcinoma had multiple granulomas, some non-caseating, epithelioid, sarcoid in type, and some showing central fibrinoid necrosis.6 An abnormal immunological response, a type IV autoimmune reaction to tumour antigens, and an unusual morphological variant have all been postulated as pathogenetic mechanisms, but they have not been thought to be associated with or related to tumour necrosis.3–5 Although Rosen refers to the possibility that “traces of fibrinoid necrosis may be found in cellular lesions”,7 and despite the single case described by Bässler and Birke in which epithelioid granulomas with central fibrinoid necrosis occurred,6 the presence of prominent necrobiotic granulomas in breast cancer appears to be rare. This may make the diagnostic consideration of infections including tuberculosis and fungal infection (possibly associated with treatment induced immunosuppression) more compelling, and may also strongly suggest the possibility of concurrent rheumatoid nodules. However, the possibility of necrotic tumour should also be a strong diagnostic consideration, as seen in cases 2 and 3, and similar to the two cases of breast carcinoma with necrotic granulomas in axillary lymph nodes described by Sethi and Carter.8 On haematoxylin and eosin staining, the morphological appearance is barely perceptible, but their recognition is facilitated by immunohistochemistry, especially using CK7 and AE1/3. Apart from a single tumour cell enveloped by an epithelioid granuloma, these non-necrotising granulomas are always adjacent to viable tumour cells, as in case 2. Necrotising granulomas associated with high grade lymphoma were reported by Hall et al.9 These authors reported two cases of high grade lymphoma containing necrotising granulomas while pointing out that non-necrotising epithelioid granulomas sometimes occur in lymph nodes replaced by lymphoma and are an integral component of a subcategory of T cell lymphoma.8 In two of the cases described here (all summarised in table 1), the granulomas were exclusively necrobiotic in type, and involved the stroma only in one case, and a draining lymph node only in another (cases 1 and 3, respectively). In case 2, sarcoid-type granulomas were present in some of the lymph nodes, with and without metastatic carcinoma, and also in the primary carcinoma, whereas two separate lymph nodes each contained a necrobiotic palisaded granuloma associated with necrotic metastatic carcinoma. In case 4 the necrobiotic granulomas were large, macroscopically visible, and present in lymph nodes without metastatic carcinoma. The patient (case 3) with stromal necrotising granulomas received chemotherapy, possibly resulting in tumour necrosis and subsequent necrobiotic granuloma formation. Morphologically, all of these necrobiotic granulomas were similar in appearance to pseudoxanthomatous nodules occurring in association with endometriosis. However, endometriosis associated lesions have typical lipofuscin containing xanthoma cells surrounding the necrotic centre and have been postulated to arise as a response to cyst contents.10 Necrobiotic granulomas have also been reported in association with non-resorbable suture material and to occur in various tissues after surgical procedures, with an idiosyncratic immune reaction to altered, necrotic collagen being the suggested aetiology.11,12
Table 1.
Details of the 4 cases reported here
| Case | Site | Type of granuloma | IHC | Diagnosis |
| 1 | 1 of 10 lymph nodes | Necrobiotic | Negative | Necrobiotic granulomatous |
| 2 | 9 of 20 lymph nodes | Necrobiotic and epithelioid granulomata (1 node); necro-biotic granulomata (1 node); epithelioid granulomata adjacent to metastases (5 nodes); epithelioid granulomata without associated metastatic carcinoma (2 nodes) | Positive | Metastatic tumour necrosis with granulomatous inflammation |
| 3 | Stroma | Necrobiotic | Positive | Tumour necrosis with granulomatous inflammation |
| 4 | 5 of 9 lymph nodes | Necrobiotic (2 nodes); epithelioid (3 nodes) | Negative | Necrobiotic granulomatous lymphadenitis |
IHC, immunohistochemistry.
“It is possible that these granulomas may result in tumour necrosis”
Therefore, this study illustrates two points. In the first instance, the pathogenesis of necrobiotic granulomas in breast cancer may differ. In two of the cases, necrobiotic granulomas were present as a direct response to necrotic tumour, in the other two cases they were not associated with tumour necrosis and may therefore represent a type IV immune response to tumour antigen. Furthermore, both responses may be present in the same patient, as demonstrated by the findings in case 2, and it is also possible that these granulomas may result in tumour necrosis. Secondly, and more importantly, their presence should prompt close morphological scrutiny and the use of CK immunohistochemistry to avoid possible underestimation of lymph node involvement.
Take home messages.
This study describes four cases of breast carcinoma with necrobiotic granulomas
In two cases, the granulomas were a direct response to necrotic tumour, whereas the other two cases were not associated with tumour necrosis and may therefore represent a type IV immune response to tumour antigen
The presence of necrobiotic granulomas should prompt close morphological scrutiny and the use of cytokeratin immunohistochemistry to avoid possible underestimation of lymph node involvement
Abbreviations
CK, cytokeratin
EMA, epithelial membrane antigen
PAS, periodic acid Schiff
ZN, Ziehl Neelsen
REFERENCES
- 1.Gorton G, Linell F. Malignant tumours and sarcoid reactions in regional lymph nodes. Acta Radiol 1957;47:381–92. [DOI] [PubMed] [Google Scholar]
- 2.Oberman HA. Invasive carcinoma of the breast with granulomatous response. Am J Clin Pathol 1987;88:718–21. [DOI] [PubMed] [Google Scholar]
- 3.Alujevic A, Juric G, Separovic V, et al. Invasive breast carcinoma with granulomatous stromal response. Zentralbl Gynakol 1997;119:343–5. [PubMed] [Google Scholar]
- 4.Santini D, Pasquinelli G, Alberghini M, et al. Invasive breast carcinoma with granulomatous response and deposition of unusual amyloid. J Clin Pathol 1992;45:885–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daroca PJ. Medullary carcinoma of the breast with granulomatous stroma. Hum Pathol 1987;18:761–3. [DOI] [PubMed] [Google Scholar]
- 6.Bässler R, Birke F. Histopathology of tumour associated sarcoid-like stromal reaction in breast cancer. An analysis of 5 cases with immunohistochemical investigations. Virchows Arch A Pathol Anat Histopathol 1988;412:231–9. [DOI] [PubMed] [Google Scholar]
- 7.Rosen PP, ed. Rosen’s breast pathology. Philadelphia: Lipincott Williams and Wilkins, 2001.
- 8.Sethi S, Carter D. Breast carcinoma associated with necrotic granulomas in axillary lymph nodes. Ann Diagn Pathol 1998;2:370–6. [DOI] [PubMed] [Google Scholar]
- 9.Hall PA, Kingston J, Stansfield AG. Extensive necrosis in malignant lymphoma with granulomatous reaction mimicking tuberculosis. Histopathology 1988;13:339–46. [DOI] [PubMed] [Google Scholar]
- 10.Carey M, Kirk ME. Necrotic pseudoxanthomatous nodules of the omentum and peritoneum: a peculiar reaction to endometriotic cyst contents. Obstet Gynecol 1993;82:650–2. [PubMed] [Google Scholar]
- 11.Alguacil-Garcia A. Necrobiotic palisading suture granuloma simulating rheumatoid nodule. Am J Surg Pathol 1993;17:920–3. [DOI] [PubMed] [Google Scholar]
- 12.Balogh K. Palisading granuloma in the kidney after open biopsy. Am J Surg Pathol 1986;10:441–2. [DOI] [PubMed] [Google Scholar]