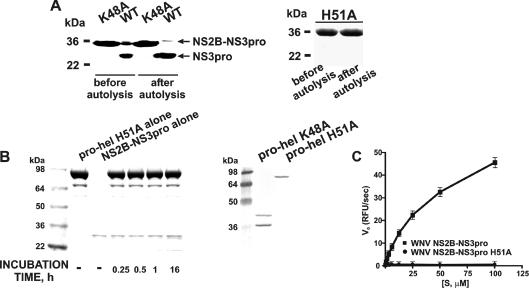
Figure 2. The properties of the mutant WNV NS2B–NS3 constructs.
(A) The NS2B–NS3pro K48A (left-hand panel) and the NS2B–NS3 H51A (right-hand panel) mutants are resistant to autoproteolysis. The purified NS2B–NS3pro (WT) and the K48A and H51A constructs (before autolysis) were incubated overnight (after autolysis). The samples were separated by gel-electrophoresis and stained with Coomassie Blue. (B) The inert NS2B–NS3pro-hel H51A construct is not cleaved by NS2B–NS3pro in trans, but the NS2B–NS3pro-hel K48A mutant is cleaved by the integral NS3pro in cis. Left-hand panel (Coomassie Blue staining), NS2B–NS3prohel H51A (pro-hel H51A) was incubated with the active WNV NS2B–NS3pro. Right-hand panel, the NS2B–NS3pro-hel K48A and H51A mutants were analysed by Western blotting of the E. coli cell lysate aliquots using the antibody the C-terminal His-tag. The individual, untagged, NS2B–NS3pro moiety is not visible on the blots. (C) NS2B–NS3pro H51A mutant does not cleave pyroglutamic acid-RTKR-7-amino-4-methylcoumarin. RFU, relative fluorescence units; Vo, the initial velocity of substrate hydrolysis; [S], substrate concentration.