Abstract
Background: The UK Medical Devices Agency has suggested that ophthalmic practitioners should, where practicable and not compromising clinical outcome, restrict corneal contact devices to single patient use to minimise a remote theoretical risk of transmission of new variant Creutzfeldt-Jakob disease (vCJD). This study reports on a modified technique of ultrasound A-scan biometry that complies with the MDA recommendations.
Methods: The right eyes of 37 consecutive hospital patients had a series of biometry readings taken with a Humphrey 820 A-scan instrument with a plane wave transducer use d conventionally and with the addition of a disposable latex cover.
Results: Intrasessional repeatability of axial length measurements was similar for conventional readings—mean difference 0.027 mm, 95% confidence intervals (CI) ± 0.44 mm and those taken with a disposable cover (0.028 mm, CI ± 0.38). Intersessional repeatability was equivalent with (0.002 mm, CI ± 0.51) and without a cover (0.03 mm, CI ± 0.51). Readings with a cover were not significantly different from those without (paired t test; p >0.05), but tended to be greater (mean difference 0.085 mm, CI ± 0.60).
Conclusions: These findings suggest that corneal contact biometry with a disposable cover is a viable and theoretically safer alternative to the conventional technique.
Keywords: biometry, repeatability, Creutzfeldt-Jakob disease
There has been extensive scientific, public, and political interest in the causative agent for transmissible spongiform encephalopathies since 1986. Reported cases of Creutzfeldt-Jakob disease in humans include familial, iatrogenic, and classic sporadic, which typically occurs in late to middle age. In March 1996 the United Kingdom reported a new variant of Creutzfeldt-Jakob disease (vCJD), which was affecting a younger age group.1 The animal transmissible spongiform encephalopathies include scrapie in sheep and goats and bovine spongiform encephalopathy (BSE) in cattle.2 As a consequence of these findings the UK government set up the Spongiform Encephalopathy Advisory Committee (SEAC). At the request of the department of health (DOH), this committee was asked to consider the possible risk of vCJD transmission through the reuse of trial contact lenses. SEAC and the DOH met with representatives of the ophthalmic professions on 28 June 1999. All present accepted that though scientific data are limited and the potential risk probably very low, as a matter of best practice, the DOH should encourage the single use of trial contact lenses. As a result, the Medical Devices Agency (MDA) issued a note3 in July 1999 for immediate action, recommending against the reuse of trial contact lenses.
The meeting also considered the question of the possible risks posed by the reuse of special complex diagnostic lenses and other instruments used in ophthalmology, which come into contact with the eye. It was agreed to refer this new issue to the deliberations of SEAC and recommend that in the mean time such devices and instruments should continue to be used. Subsequently, acting on SEAC recommendations, MDA Advice Notice 1999(04)4 was issued on 21 October 1999 entitled “Single patient use of ophthalmic medical devices: implications for clinical practice.” This stated, “As far as devices that touch the surface of the eye are concerned, practitioners should restrict these to single patient use wherever practicable and where this does not compromise the clinical outcome.” This casts some doubt on the role of corneal contact procedures in research, where a procedure may be performed that is not forming a part of a subject's clinical management. The question has been posed “In what areas of practice can we develop and afford to introduce disposable instrumentation and devices on a routine basis . . .?”5 Clearly alternatives need to be sought where corneal contact is to take place. Single use applanation tonometer probes are already commercially available at a reasonable cost. At the time of writing the equivalent is not available for corneal contact A-scan biometry. For this study we therefore chose to investigate the repeatability and validity of A-scan biometry measurements taken using a Humphrey 820 A-scan ultrasound biometer in conjunction with a single use disposable protective cover, currently commercially available for use with the Tonopen tonometer. The possible influence of cover thickness was also investigated.
SUBJECTS AND METHODS
A-scan technique
Thirty seven consecutive patients, mean age 72 (range 36–95) years, attending a hospital cataract centre for preassessment had, as part of their clinical management, a routine A-scan biometry measurement taken on both eyes, using a Humphrey 820 A-scan biometer with a plane wave transducer, in automatic mode. This was followed immediately by, on the right eye only, a repeat measure (study reading 2) and two measurements (readings 3 and 4) using an Oculofilm single use disposable Tonopen cover fitted over the biometer probe (Fig 1). These covers are manufactured by Solano Ophthalmic Products, Jacksonville, FL, USA, and distributed in the United Kingdom by Carleton Optical Equipment Ltd. All measurements were taken by one investigator (KC) on a single apparatus to minimise interobserver error.6 The fixation target was a spotlight at 6 metres rather than the probe's internal fixation light which has limitations for some biometric measurements.7 Omitted from the data collection process were any patients attending following the use of miotics (n = 2), aphakic extended wear contact lens wearers requiring secondary implants (n = 2), and patients exhibiting very poor fixation which prevented full data being collected (n = 3).
Figure 1.
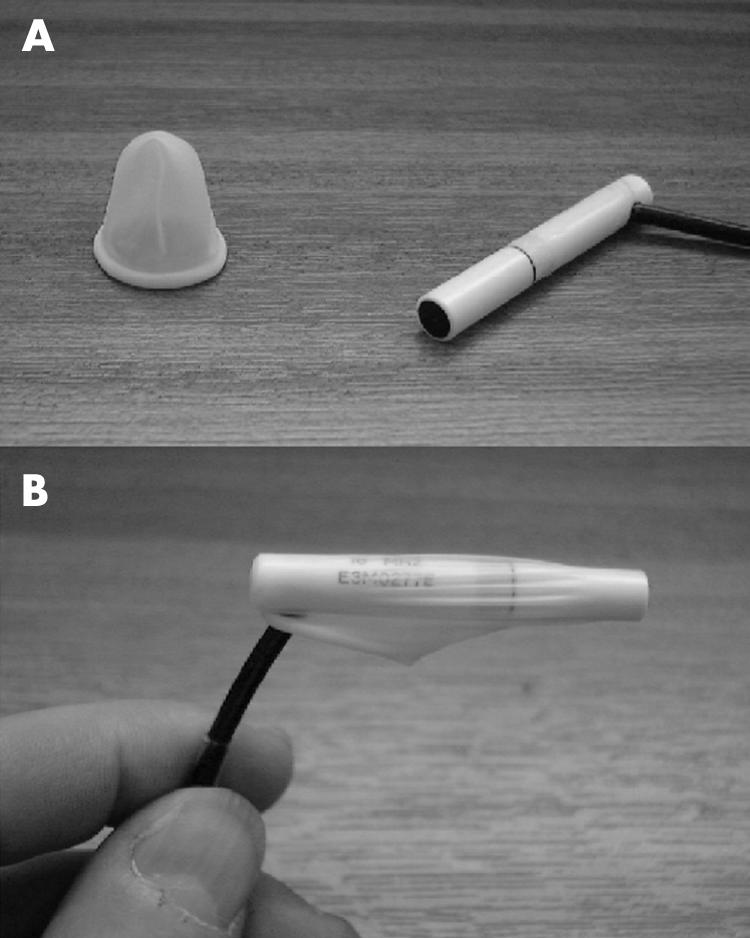
(A) “Oculofilm” disposable cover in unstretched form shown adjacent to the Humphrey ultrasound biometer model 820 transducer probe. (B) The oculofilm disposable cover shown in situ, stretched over the biometer probe.
The biometer probe required two applications of viscous coupling solution, one before and a second after the placement of the disposable cover, to obtain an adequate signal. Care was also taken to ensure a reservoir of fluid was not held beneath the cover by shaking off excess coupling fluid before placement of the cap and squeezing out any remaining reservoir once the cap was in place. This was achieved by pressing the protected biometer probe against the sterile cap mount. There was no noticeable attenuation of the signal. It was not necessary to increase the signal gain from the 60% default value.
In automatic mode the Humphrey 820 model only accepts measurements that meet its programmed criteria for consistency and amplitude. These are presented as a frozen display and the operator is warned by an auditory signal. In the current study all “frozen” readings were accepted by depressing the instrument foot pedal once. Five such readings were accrued and stored. On obtaining five readings the instrument would automatically compute an average after discarding the lowest two stored axial length measurements. If an error signal was obtained (signified by the appearance of “The axial length criteria not met” message) the investigator would review the stored measurements and traces and discard any doubtful traces and extreme readings. Replacement readings were then taken. At the conclusion of the preassessment appointment a final measurement was taken both without (study reading 5) and with the disposable cover (reading 6) in order to obtain an intersessional comparison of readings.
Statistical analysis
Our data are represented in the now widely accepted Bland and Altman style.8 The difference between the two measures plotted versus the mean represents the degree of agreement, 95% of the differences between the two measurements fall within the confidence limits which are calculated from the mean difference ± 1.96 × standard deviation of differences.9 This allows any bias between the two methods to be viewed throughout the recorded range. This method was preferred to correlation coefficients as the measure of correlation is not necessarily a measure of agreement.10 If, for example, a second method exactly doubles each recorded measure using method one there will be perfect correlation but no agreement. Finally, any bias was assessed statistically as the mean of differences compared to zero (t test).
Disposable cover parameters
On completion of the clinical data collection, two separate investigations into the mean thickness and within batch variability of the disposable covers were carried out on covers from the same batch as used in the main study. The first of these applied an optical technique involving travelling microscopy in order to provide data on the predicted maximum thickness with the cover is in its dry non-stretched state. Initially, two observers recorded their interpretation of 10 separate readings using a travelling microscope's vernier measuring scale blind to their colleague's results. Subsequently, a disposable cover had a vertical cut made in its end and was then mounted so as to enable the unstretched thickness to be measured using the travelling microscope. The two observers then each took five repeated measures of the thickness. The procedure was repeated for a further nine covers, making a total of 10 covers. The mean thickness and variability of the covers, intraobserver variability, and intraobserver agreement on vernier measures were then analysed using a series of paired t tests.
Since readings from covers in an unstretched state presumably represent a greater thickness than that actually obtained during the data collection process two more sophisticated measurement systems—laser interferometry and coordinate measurement—were used to gain information on their thickness when stretched in a manner similar to the conditions under which they were used. For these methods it was necessary to manufacture a dummy transducer from silver steel that was then clamped into position for use with the Hewlett Packard model HP5529A laser interferometer and also the LKG90-C coordinate measuring machine (Fig 2). Both techniques are capable of resolving thickness to 1/10th μm (0.0001 mm).
Figure 2.
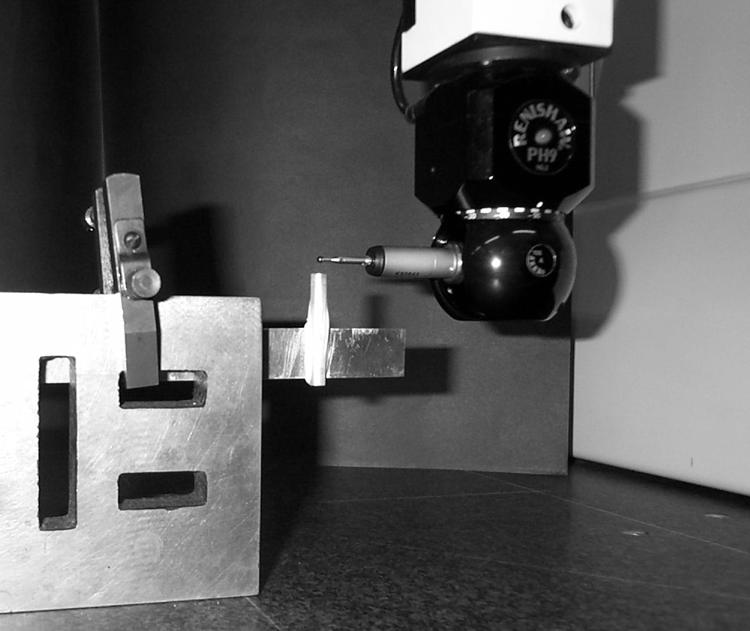
Close up view of the dummy transducer with disposable cover in place mounted on the coordinate measurement test rig during a measurement.
In the interferometric technique the position of the end of the dummy transducer in relation to the laser was measured, with the assistance of “dynamic calibrator” computer software, and the measurement gauge set to zero before placement of the disposable cover. After wetting the transducer and placing a disposable cover over it a further application of coupling solution was used on the end of the cover, as had been performed during the data collection. The position of the end of the covered transducer in relation to the laser was measured 10 times, the change in the distance, as recorded by the gauge (that is, half the change in path length), being equal to the thickness of the disposable cover. After carefully removing the cover the measurement gauge of the instrument was rechecked to ensure that the position of the dummy probe had not changed during positioning or removal of the cover. The procedure was repeated for nine further disposable covers. An equivalent method was used for the coordinate measurement technique with the constraint that the outer surface of the cover could not be wetted. A further four samples taken from different batches were subjected to the same travelling microscopy and coordinate measurement techniques in order to provide data on interbatch variability.
RESULTS
Reliability and validity
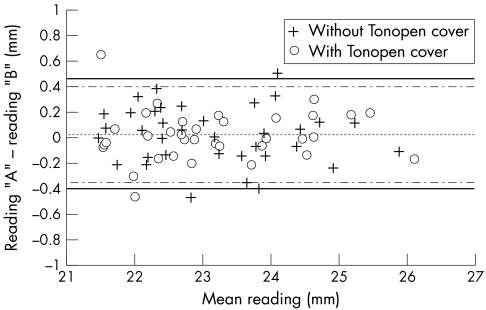
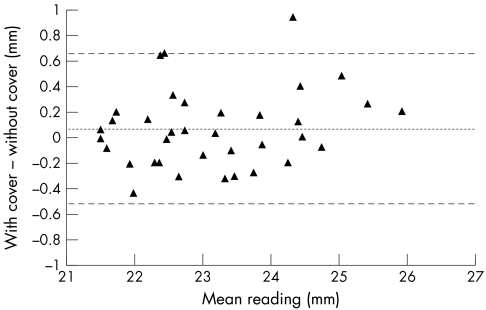
Analysis of the Bland and Altman8 plot of the repeatability data (Fig 3) illustrates the even distribution of the data points for repeated measures throughout the measured range regardless of whether or not a protective cover was used. When comparisons are made of the measures with and without the cover the mean difference and 95% confidence intervals do, however, increase to 0.1 mm and CI ± 0.6 mm respectively for the intrasessional validity comparisons (Fig 4 and Table 1). The bias seen is for slightly longer readings (on average 0.085 mm) to be obtained with the disposable cover in place, therefore suggesting that the thickness of the disposable cover does perhaps have some influence on the measurement of axial length.
Figure 3.
Intrasessional repeatability of readings taken without (crosses) and with (open circles) the disposable cover during session 1. Difference data are plotted versus mean data. Reading “A” indicates the first and “B” the second of a pair. The overall mean differences were virtually identical for each condition (centre dotted line), the outer lines represent 95% confidence limits without (solid line) and with the cover (broken line). Table 1 summarises numerical data relating to this figure.
Figure 4.
Intrasessional validity of readings taken during session 1. The data are computed from the first of each pair of readings. The centre line represents the mean difference and the outer lines the 95% confidence limits. A slight preponderance of positive data (ordinate) indicates a tendency for longer readings being obtained with the disposable cover in place. Table 1 summarises numerical data relating to this figure.
Table 1.
Mean of differences, 95% confidence intervals, and results of paired t tests for both repeatability data, with and without a disposable (Tonopen oculofilm) cover and the validity of measurements with the disposable cover
| Condition | Mean difference (mm) | 95% confidence intervals (mm) | t test result |
| Intrasessional repeatability without cover | 0.027 | ± 0.44 | p=0.20 |
| (reading 1 − reading 2) | NS | ||
| Intrasessional repeatability with cover | 0.028 | ± 0.38 | p=0.59 |
| (reading 3 − reading 4) | NS | ||
| Intrasessional validity | 0.07 | ± 0.59 | p=0.82 |
| (reading 3 − reading 1) | NS | ||
| Intrasessional validity | 0.10 | ± 0.60 | p=0.37 |
| (reading 6 − reading 5) | NS | ||
| Intersessional repeatability without cover | 0.03 | ± 0.51 | p=0.20 |
| (reading 1 − reading 5) | NS | ||
| Intersessional repeatability with cover | 0.002 | ± 0.51 | p=0.72 |
| (reading 3 − reading 6) | NS |
NS = not significant.
Study reading 1= initial uncovered; 2 = repeat uncovered; 3 = initial covered; 4 = repeat covered; 5 = final uncovered; 6 = final covered.
In order to assess the variation in any repeated measure, paired t tests were carried out for intrasessional repeatability of the biometry measures with and without the disposable cover using the initial paired readings. T tests on intersessional measures compared the first biometry measure from session one with and without the disposable cover with the respective measure in the final session. To validate the biometry measures with the disposable cover t tests were then performed comparing the first measure without the disposable cover and the first measure with the disposable cover. None of these t tests found the distribution of the obtained readings to be significantly different at the p = 0.05 significance level (Table 1). This lack of significance suggests very little variation in the repeated biometry measures regardless of whether they were taken either with or without the disposable cover. The mean difference of repeated measures remained in the order of ≅ 0.03 mm for both the intrasessional and intersessional conditions.
Tonopen cover thickness and observer agreement
The mean thickness of 10 unstretched Tonopen covers from the main study sample was found to be 0.069 mm, CI ± 0.020 mm (Table 2). The overall range for this sample extended from 0.055 mm to 0.083 mm. The mean of the differences between the two observers was 0.003 mm. A two tailed t test comparing the readings of the two observers found no statistical difference (p = 0.14). When assessing the agreement of two observers repeatedly recording the same vernier reading, on four of 10 occasions the observers were in exact agreement with each other, while three readings differed by 0.01 mm and three by 0.02 mm—that is, the mean difference was 0.009 mm CI ± 0.0018 mm. The mean unstretched thickness ranged between 0.069 and 0.088 mm for the remaining four batches (Table 2).
Table 2.
Summary of average thickness readings (and 95% confidence intervals), in unstretched and stretched state, for samples of 10 disposable oculofilm covers derived from five separate batches
| Unstretched readings (mm) | Stretched readings (mm) | ||
| Batch study sample number | Travelling microscopy (CI) | Coordinate (CI) | Interferometry (CI) |
| 1 (used in main study) | 0.069 (0.020) | 0.013 (0.0012) | 0.012 (0.0029) |
| 2 | 0.079 (0.020) | 0.010 (0.0008) | – |
| 3 | 0.080 (0.025) | 0.008 (0.0008) | – |
| 4 | 0.088 (0.024) | 0.009 (0.0018) | – |
| 5 | 0.069 (0.018) | 0.010 (0.0024) | – |
When the disposable covers were placed on the dummy transducer in a scenario most likely to replicate the actual conditions under which data were collected the mean thickness of the 10 disposable covers in our study sample was 0.012 mm (12 μm) with CI ± 0.0029 mm (2.9 μm). The range extended from 0.009 to 0.019 mm.
The results of the coordinate measuring technique were encouragingly close at 0.013 mm (13 μm) but with a greatly reduced confidence intervals 0.0012 mm (1.2 μm). The latter therefore became our first choice method for the remaining four batches. Details of these batches, both unstretched and stretched, and the results of the interferometry technique used on the main study sample are summarised in Table 2.
DISCUSSION
Humphrey Instruments claims an inherent instrument accuracy of ± 0.034 mm for the biometer model 820 in their instrument specification literature and a patient measurement accuracy of ± 0.10 mm. Our largest mean difference for repeatability and reproducibility was in the order of 0.03 mm and as such compares favourably with the optimum accuracy. Two other groups11, 12 have reported repeatability for the Humphrey 820 biometer. The two experimenters in Rudnicka's11 study produced absolute mean differences of 0.42 mm and 0.008 and 95% confidence intervals in the order of ± 0.25 mm and ± 0.20 mm, respectively, when considering the data of both eyes. Zadnik and coworkers12 did not report directly on axial length but their separate data produced 95% confidence intervals of ± 0.29 mm for anterior chamber depth, ± 0.20 mm for lens thickness, and ± 0.37 mm for vitreous chamber depth. The earlier studies differ in their methodology—one using a slit lamp mounted probe,11 the other a hand held approach similar to that used in our study.12 This may explain the closer resemblance of results from the two studies using the handheld probe. The pragmatic nature of this investigation and its sample size mean that there are no very short or very long eyes represented in the study population. The available data do not, however, suggest that the axial length of individual eyes is of relevance to the findings.
Both the previous studies differ from the present study by using subjects free of pathology, which we presume would give less variation in the reflected wave.13 In view of the current MDA advice our study population were, of necessity, restricted to patients undergoing biometry as part of their standard clinical investigations. Forty three per cent of the eyes measured for the study had a best corrected visual acuity of 6/24 or worse. Forty eight per cent of the patients had a best corrected acuity in the left, fixing eye, of 6/24 or worse with an overall range of acuities from 6/5 to hand movements, which may have had a detrimental effect on the fixational stability of the eye. It was considered, however, that the use of a distance spotlight as the fixation target throughout the study would help to reduce the impact of any such instability. Since the standard clinical protocol during ultrasound biometry would not normally include correction of ametropia, the levels of vision experienced in the fixing eye of some “ophthalmologically normal” observers during the procedure may not differ from those of our observers. With the exception of the three patients who were excluded from the study on account of very poor fixation, no difficulty was noted in obtaining readings from our “typical” clinical population, either with or without the disposable cover in situ. Considering all of the study population, readings were obtained with equal ease whether or not a cover was in use during measurement.
Whereas the average unstretched thickness of the disposable covers was equivalent to 90% of the mean discrepancy between readings taken with and without the cover, the average stretched thickness of the covers in the simulated interferometric test rig was equal to only 12% of this difference. The extent to which the oculofilm covers thin (to an average of 10 μm) compared with their original dry state (average 77 μm) when wet and stretched over the dummy transducer was a source of initial surprise. This change may be due to a reduction in the friction between the latex cover and dummy probe on wetting the inner surface. It is also apparent from Table 2 that the thickness of the stretched covers cannot readily be estimated from the unstretched thickness as when ranked in order of thickness unstretched the covers do not correspond with the ranking when stretched. One would assume the cover surface profile would be a discontinuous surface of peaks and troughs; hence, as the coordinate measurement takes a reading the final recorded thickness will be highly dependent on the region being measured be it a peak or a trough. Perhaps the cover could have been removed and replaced between each measurement to overcome this problem, though it is unclear as to whether this would have ultimately strained the cover, weakening the polymer and giving rise to an unnaturally thin reading or perhaps even damaging the cover, preventing collection of the full set of measurements.
The possibility of the 0.085 mm increase in axial length being related to the properties of ultrasound transmission through latex was considered. The speed of sound in latex rubber is in the order of 1550 m/s (± 10 m/s)14 compared with the 820 biometer's assumed speed of 1532 m/s for vitreous and aqueous. One would not therefore anticipate an appreciable increase in the measured axial length with the latex cover in place over and above the actual cover thickness, in fact when using a typical cover of 10 μm this would result in an increase of 0.11 μm to the cover thickness.
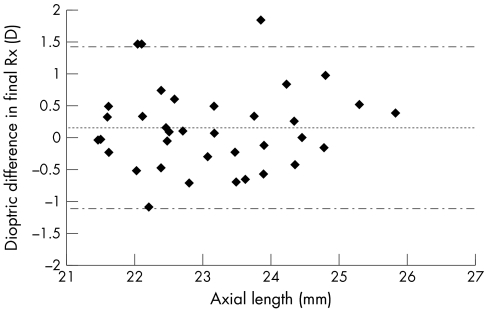
The clinical implications of our finding of a marginal increase in measured axial length with the disposable cover was investigated by inputting the data into a formula used to predict the necessary intraocular lens (IOL) power required to achieve the relevant level of ametropia in individuals undergoing cataract surgery. The SRK/T formula15, 16 incorporated into the on-screen software of the biometer used in this study requires the input of corneal radii of curvature, axial length, and desired optical prescription. If other parameters remained constant, leaving the axial length differences as the only variable when applying this formula, a shift towards hyperopia in the order of 0.16 dioptres (CI ± 1.27D) occurs (Fig 5). This is, of course, smaller than the power increments for a typical IOL, usually 0.50D. Omission of the three readings falling outside the confidence intervals results in a mean difference of only +0.03D. The differences in refractive error found based on the two axial length readings are in fact independent of the magnitude of the patients' keratometry readings, providing the keratometry readings remain constant for each of the two calculations. The tendency for a bias in producing marginally elongated axial lengths is therefore unlikely to be of significance clinically, being similar to the precision of normal subjective refractive technique.17, 18 Clinical outcome in the selection of IOL power should not be compromised by use of the raw data obtained with the cover in place. The lack of any statistically significant difference in our readings obtained with and without the cover also supports this opinion.
Figure 5.
Dioptric differences in final optical prescription findings resulting from substituting conventionally obtained axial length readings in the SRK/T formula with those derived from the modified procedure. The slight preponderance of positive data (ordinate) signifies a tendency for individuals to be left relatively hyperopic compared with the conventional readings. The data originate from the same data set presented in Figure 4. The centre dotted line represents the mean difference and the outer broken lines the 95% confidence limits.
The use of a disposable cover is not the only method of avoiding direct corneal contact when undertaking ultrasound biometry. A further possible technique, which is of particular value in the examination of unanaesthetised infants, is to conduct through the lid biometry. Two such methods have previously been described, each requiring the operator to successfully locate the corneal bulge beneath the closed eyelid in order to obtain readings.19, 20 These require digitisation of the A-scan hard copy, followed by either remeasuring from the retina to the beginning of the anterior chamber19 or measuring from the first echo to the final lid echo which is then subtracted from the axial length measurement generated by the biometer.20 This method is clearly more complicated and not without its difficulties as typical biases of 0.1–0.2 mm have been reported compared with the corneal technique.20 An alternative non-invasive biometric method makes use of the optical technique of partial coherence interferometry.21 Although early reports suggest commercially available versions of such technology (Carl Zeiss, IOL-Master) show promise compared with traditional ultrasound biometry,22 this method has yet to be fully evaluated.
CONCLUSIONS
Our findings suggest that corneal contact biometry with a disposable “Oculofilm” cover applied to a plane wave transducer is a viable alternative to the conventional technique that would comply with the recommendations of MDA Advice Notice 1999(04) by restricting the contacting surface of the device to single patient use. The unit cost of the cover is sufficiently inexpensive to encourage use of this theoretically safer method.
Acknowledgments
The Oculofilm covers used in this study were donated by Mr Nick Fitzig of the UK distributor, Carleton Optical Equipment, Carleton House, 549 Eskdale Road, Uxbridge, Middlesex, UB8 2RT, UK. The authors would also like to thank John Simpson for construction of the dummy biometer probe and Dr Mel Burdekin and Mr C K Lim from the Manufacturing Division Department of Mechanical Engineering, UMIST, for assistance with the coordinate and laser interferometric measurements.
REFERENCES
- 1.Will RG, Ironside JW, Zeidler M, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 1996;347:921–5. [DOI] [PubMed] [Google Scholar]
- 2.Cox MA. A systematic review of the published literature review to identify the methods of inactivation for unconventional agents of transmissible spongiform encephalopathies. 1998. Available at http://www.medical-devices.gov.uk.
- 3.MDA Advice Notice `Use of trial contact lenses on multiple patients' available at http://www.medical-devices.gov.uk/an1999(02).htm
- 4.MDA Advice Notice `Single patient use of ophthalmic medical devices; implications for clinical practice' available at http://www.medical-advices.gov.uk/an1999(04)htm
- 5.Tullo AB, Buckley RJ, Painter M. CJD and the eye (editorial) Eye 2000;14:259–60. [DOI] [PubMed] [Google Scholar]
- 6.Longstaff S. Factors affecting intraocular lens power calculation. Trans Ophthalmol Soc UK 1986;105:642–6. [PubMed] [Google Scholar]
- 7.Steele CF, Crabb DP, Edgar DF. Effects of different ocular fixation conditions on A-scan ultrasound biometry measurements. Ophthal Physiol Opt 1992;12:491–5. [PubMed] [Google Scholar]
- 8.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- 9.Bland JM. An introduction to medical statistics. Oxford: Oxford University Press, 1996:123–5.
- 10.Zadnik K, Mutti DO, Bullimore MA. Use of statistics for comparing two measurement methods. Optom Vis Sci 1994;71:539–40. [DOI] [PubMed] [Google Scholar]
- 11.Rudnicka AR, Steele CF, Crabb DP, et al. Repeatability, reproducibility and intersession variability of the Allergan Humphrey ultrasonic biometer. Acta Ophthalmol 1992;70:327–34. [DOI] [PubMed] [Google Scholar]
- 12.Zadnik K, Mutti DO, Adams AJ. The repeatability of measurement of the ocular components. Invest Ophthalmol Vis Sci 1992;33:2325–33. [PubMed] [Google Scholar]
- 13.Storey JK. Measurement of the eye with ultrasound. Ophthal Opt 1982;22:150–60. [Google Scholar]
- 14.Weast RC. Handbook of chemistry and physics. Cleveland: CRC Press, 1975.
- 15.Sanders DR, Retzlaff JA, Kraff MC, et al. Comparison of the SRK/T formula and other theoretical and regression formulas. J Cataract Refract Surg 1990;16:341–6. [DOI] [PubMed] [Google Scholar]
- 16.Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg 1990;16:333–40. [DOI] [PubMed] [Google Scholar]
- 17.Gullion M. Automated refraction in aphakia-II. Its repeatability and accuracy compared to conventional techniques. Ophthal Physiol Opt 1986;6:85–9. [DOI] [PubMed] [Google Scholar]
- 18.Bullimore MA, Fusaro RE, Adams CW. The repeatability of automated and clinician refraction. Optom Vis Sci 1998;75:617–22. [DOI] [PubMed] [Google Scholar]
- 19.Laws F, Laws D, Wood I, et al. Assessment of a new through-the-eyelid technique for `A' scan ultrasound ocular axial length measurement. Ophthal Physiol Opt 1998;18:408–14. [PubMed] [Google Scholar]
- 20.Twelker JD, Kirschbaum S, Zadnik K, et al. Comparison of corneal versus through-the-lid A-scan ultrasound biometry. Optom Vis Sci 1997;74:852–8. [DOI] [PubMed] [Google Scholar]
- 21.Drexler W, Findl O, Menapace R, et al. Partial coherence interferometry: a novel approach to biometry in cataract surgery. Am J Ophthalmol 1998;126:524–34. [DOI] [PubMed] [Google Scholar]
- 22.Haigis W, Lege B, Miller N, et al. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol 2000;238:765–73. [DOI] [PubMed] [Google Scholar]