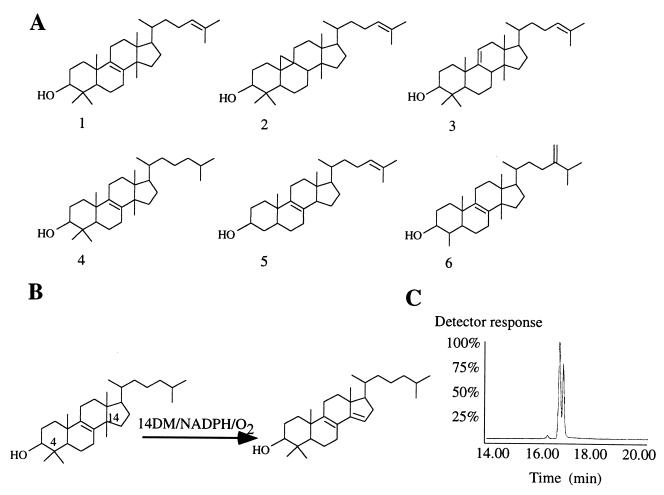
Figure 1.
(A) Sterol molecule structures. 1, lanosterol; 2, cycloartenol; 3, parkeol; 4, DHL; 5, zymosterol; and 6, obtusifoliol. (B) DHL 14α-demethylation. Conversion of DHL to 4,4-dimethyl-5α-cholesta-8,14-diene-3β-ol in the presence of MT P45014DM, NADPH, and molecular oxygen. (C) GLC profile of overnight conversion of 2 mg DHL. E. coli Fld/Fdr system was used as P450 electron donor. The peaks at 16.38 and 17 min correspond to the DHL and metabolite retention times, respectively.