Abstract
Background/aim: Iris nodules are an uncommon clinical sign in uveitis. The diseases most commonly associated with iris nodules and uveitis include sarcoidosis, Vogt-Koyanagi-Harada syndrome, multiple sclerosis, Fuchs’ heterochromic iridocyclitis, and metastatic infection. While many of these diseases may be appropriately treated with immunosuppressive medication, the management of infectious uveitis is antimicrobial therapy. Inappropriate immunosuppressive therapy may result in a poor outcome for the patient with an infection. Consequently, cases of uveitis with iris nodules were reviewed to identify clinical features that may help differentiate infection from non-infectious inflammation.
Methods: The clinical database of 1353 consecutive patients evaluated at a tertiary care referral based North American uveitis clinic were retrospectively reviewed to identify cases of infectious uveitis with iris nodules. A Medline search was performed to identify additional cases. From these cases information regarding clinical presentation, diagnosis, treatment, and outcome were collected.
Results: Three cases (three eyes) were identified from the authors’ own records of infectious uveitis with iris nodules. An additional 25 cases of infectious uveitis with iris nodules were identified in 22 published reports. Analysis of the authors’ cases and these reports showed that infectious uveitis with iris nodules was specifically characterised by some or all of the following: (1) creamy, soft appearance to the nodule(s), (2) unilateral disease, (3) persistence or growth of the nodule(s) despite corticosteroid therapy, (4) marked inflammatory response in the anterior chamber and/or vitreous humour, and/or (5) history suggesting a potential source of septic emboli.
Conclusion: Certain features of the clinical history and examination are useful in the diagnosis of metastatic infection in patients presenting with uveitis and iris nodules.
Keywords: iris nodules, infectious uveitis
Uveitis of infectious or non-infectious aetiology may initially present with a similar clinical picture. However, the appropriate treatment of infectious uveitis is very different from treatments that are indicated for other forms of uveitis. The immunosuppressive therapy commonly used to treat non-infectious uveitis may prolong or worsen an infection. Thus, accurate and timely diagnosis of an infectious uveitis is essential in providing appropriate patient care.
Iris nodules are an uncommon finding in uveitis. When seen, they are thought to be suggestive of specific aetiologies of a uveitis such as sarcoidosis, Vogt-Koyanagi-Harada syndrome, multiple sclerosis, Fuchs’ heterochromic iridocyclitis, particularly in darkly pigmented individuals, and infectious uveitis.1–4 Several bacterial species have been associated with uveitis and iris nodules including Mycobacterium,5–7 Treponema,8,9 and Rickettsia.10 Additionally, many fungal agents, such as Cryptococcus,11 coccidioidomycosis,12–15 and Candida,16 also have reported associations with iris nodules.
We present three patients with iris nodules found in association with uveitis in which a specific infectious organism was identified. In each case, the uveitis was initially diagnosed as non-infectious. In one case, the isolated organism was a Corynebacterium species, an organism that has not previously been reported in association with iris nodules. Additionally, a literature review was conducted using Medline to identify previous reports of infectious uveitis with iris nodules. Our cases and the published literature show that a thorough history, detailed examination, and the use of appropriate diagnostic techniques can be invaluable in the prompt and accurate recognition of infection as a cause of uveitis with iris nodules.
METHODS
We examined the clinical database maintained at the uveitis service of the Oregon Health and Science University (OHSU) over a 16 year period from September 1985 to November 2001, to identify cases of infectious uveitis with iris nodules. Of a total of 1353 patients with uveitis, three patients (three eyes) were diagnosed with this condition. In other words, the prevalence of uveitis with iris nodules for our tertiary referral uveitis practice was 0.22% of all uveitis patients. One of these cases has been previously reported in the form of a photographic essay.17 From the medical records of these three patients we recorded clinical information which included mode of presentation, method of diagnosis, the responsible organism, source of infection, treatment, and final outcome. A Medline search (key words: iris nodules, iris mass, iris nodules and uveitis, iris mass and uveitis, uveitis, and nodules) was performed to identify additional cases and collect similar information. This search included the entire memory of the Medline database from 1966 up to November 2001.
RESULTS
Case 1
An 81 year old white male was referred to the uveitis service at OHSU with a 3 week history of a left granulomatous uveitis with an iris mass. The uveitis had been refractory to topical corticosteroid therapy. Ocular history was remarkable only for uncomplicated cataract surgery on both eyes several years previously. His medical history was notable for coronary bypass and aortic valve replacement surgery with a bovine valve 4 months before the onset of the uveitis. His postoperative course had been complicated by a sternal wound infection from which a Candida species had been cultured. The infection had been treated with intravenous antifungal agents. He had noted no drainage of the wound for 2 months but reported fevers and chills over the 4 weeks before his referral.
On examination, his best corrected visual acuity was 20/20 in the right eye and 20/40 in the left eye. Intraocular pressures were 10 mm Hg and 26 mm Hg in the right and left eyes, respectively. Examination of the right eye was normal, but the left eye had 2+ cell in the anterior chamber with keratic precipitates and a large, fluffy iris nodule (Fig 1). There were 1+ vitreous cells and vitreous membranes in the left eye, affording a hazy view of the fundus.
Figure 1.
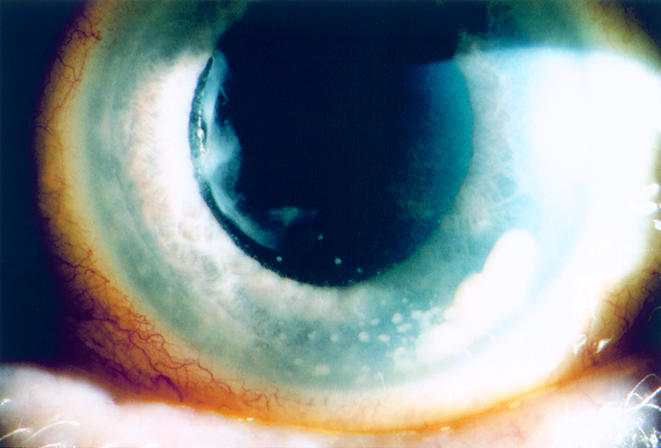
Case 1. A fluffy white iris nodule is visible from the 3:30 to 5:30 clock hours position in the left eye. Associated keratic precipitates are also seen. The nodule was partially aspirated in the clinic and found to be firmly adherent to the iris face. Left visual acuity was 20/40.
A metastatic infection was suspected and an anterior chamber paracentesis, with aspiration of a portion of the iris nodule, was performed. The patient was admitted to the hospital for blood cultures and parenteral antibiotics. He was found to be febrile on admission. While blood cultures were negative, the aqueous aspirate grew Candida albicans and treatment was begun with intravenous fluconazole. A culture of the sternotomy wound was also positive for C albicans. Further tests including a transoesophageal echocardiogram, computed axial tomogram scan of the chest, and tagged white blood cell scan failed to reveal any other source of infection.
After treatment with fluconazole, the patient’s fevers resolved, but best corrected visual acuity in the left eye worsened to 20/100 and increased vitreous inflammation was noted. A left pars plana vitrectomy with injection of amphotericin B and debulking of the iris nodule was performed. Cultures of the vitreous fluid grew C albicans. Postoperatively, his left visual acuity improved to 20/25 and intraocular pressure stabilised at 17 mm Hg. The iris nodule and anterior chamber inflammation were resolved after 3 months of treatment (Fig 2).
Figure 2.
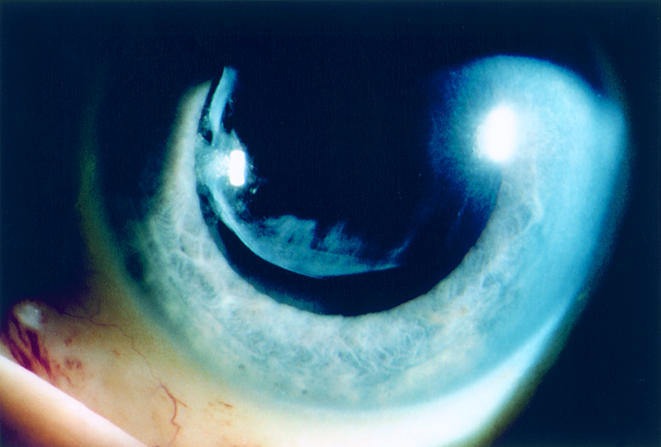
Case 1. Resolution of the anterior chamber reaction, keratic precipitates, and iris nodule is apparent 2 months after vitrectomy and injection of intraocular amphotericin B. Final left visual acuity was 20/25.
Case 2
A 41 year old white female was diagnosed with uveitis in her right eye and treated for 12 days with topical and oral corticosteroid without improvement. She developed elevated intraocular pressure which responded to a combination of topical timolol, brimonidine, and dorzolamide. Results of laboratory studies, including a purified protein derivative skin test, complete blood count, erythrocyte sedimentation rate, and chest x ray, were normal. Her medical history was unremarkable and she had no systemic symptoms. She was referred to the OHSU uveitis service for further evaluation.
Her best corrected visual acuity on examination was 20/25 on the right and 20/20 on the left. Right intraocular pressure was controlled at 13 mm Hg. On slit lamp examination of the right eye, there were keratic precipitates, 3+ aqueous cell, and a heavy aqueous flare with fibrin clot formation. There were five discrete nodules on the inferior iris (Fig 3A and B). The posterior pole was normal. Examination of the left eye was normal.
Figure 3.
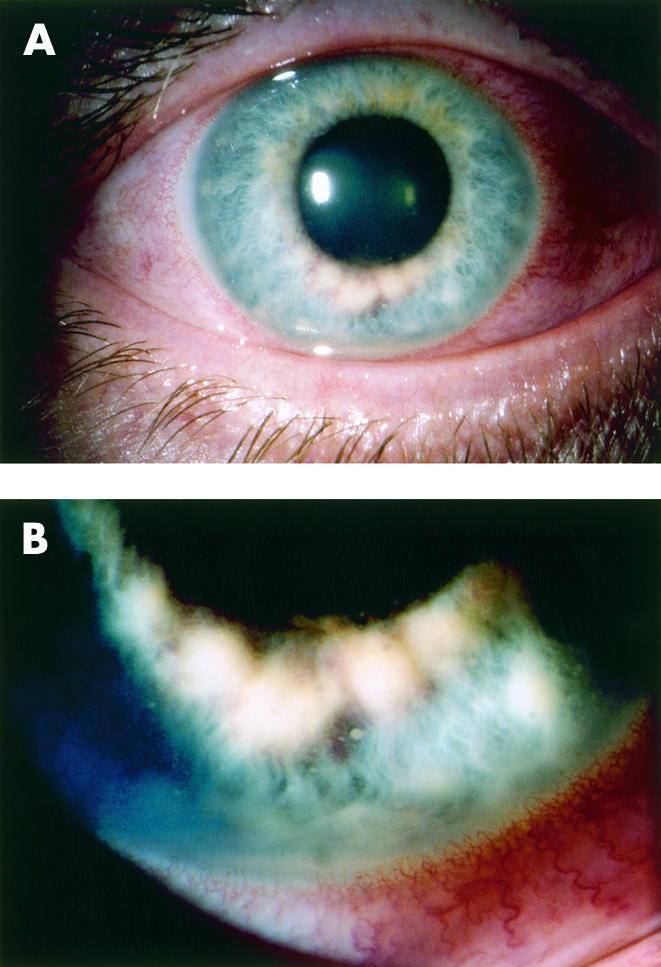
(A) Case 2. Iris nodules seen at the inferior pupillary border of the right eye show a creamy intrastromal appearance. Right visual acuity was 20/25. (B) Higher magnification view of the nodules shown in (A) demonstrates five discrete nodules with obvious anterior chamber reaction.
After an anterior chamber paracentesis, she was admitted to the hospital to search for a systemic source for a suspected intraocular infection. Cultures of the aqueous aspirate grew a Corynebacterium species and she commenced treatment with vancomycin. No systemic source of infection was identified, and the patient was discharged on intravenous vancomycin. After 2 months of therapy, including topical corticosteroid, her symptoms had resolved and her vision had returned to 20/15 in the right eye (Fig 4).
Figure 4.
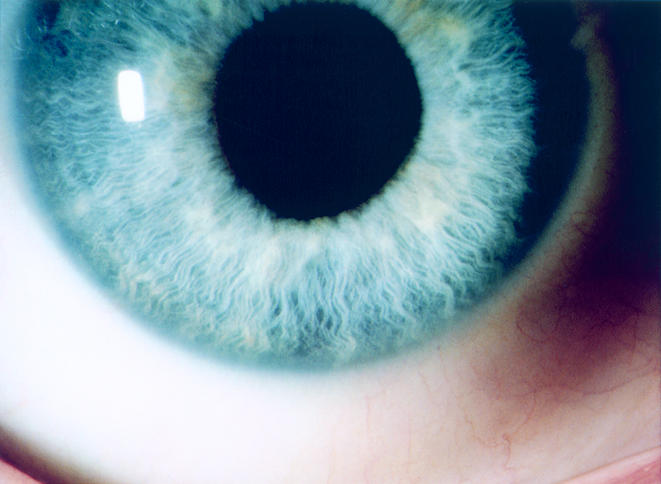
Case 2. There is complete resolution of the inflammation and iris nodules in the right eye following 2 months of parenteral antibiotics. Final right visual acuity was 20/15.
Three years later, the patient had a recurrence of her right iritis without iris nodules. She had since moved from our referral area. No cultures were taken, and her disease was treated with topical corticosteroid and a cycloplegic. There was complete resolution of her symptoms and signs within 4 weeks.
Case 3
A 60 year old white female noted 3 weeks of left eye pain, redness, and photophobia. Her symptoms had worsened on topical and periocular corticosteroid therapy which had been commenced for a presumed non-infectious left anterior uveitis. Further, after 1 week of corticosteroid therapy she developed a large white iris nodule which appeared to arise from the iris stroma (Fig 5). She had a medical history remarkable for coronary bypass surgery with mitral valve replacement, performed 6 months before her referral. She was referred to the uveitis clinic at OHSU for further evaluation. On specific questioning she described recent onset of arthralgia in her right first metatarsal with associated redness and swelling.
Figure 5.
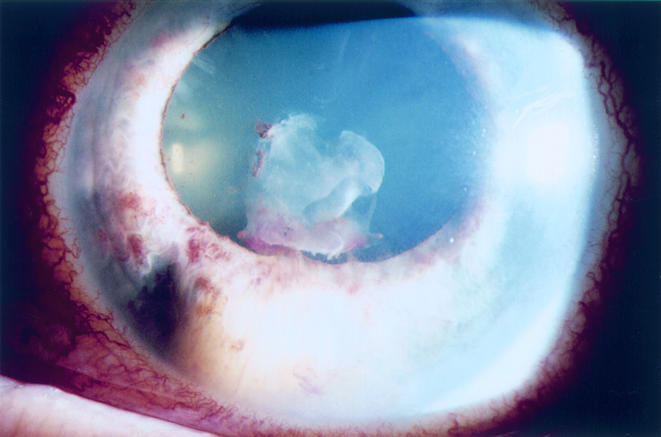
Case 3. A creamy white intrastromal iris nodule is seen from the 6:30 to 8:00 clock hours position in the left eye. There is also fibrinous exudate on the anterior lens capsule. Left visual acuity was 20/80.
Her visual acuity was 20/40 in the left eye and left intraocular pressure was normal at 13 mm Hg. On slit lamp examination she was noted to have fine keratic precipitates, 1+ aqueous cell, and a large iris nodule in the left anterior segment. Left fundus examination was normal. Examination of the right eye was likewise normal. She also had a splinter haemorrhage on the nail bed of her right index finger, and a tender nodule on her right forearm.
Anterior chamber paracentesis was performed, and the patient was admitted to the hospital. The aqueous culture was positive for Actinobacillus actinomycetemcomitans, an organism known to cause infectious endocarditis.18,19 Skin biopsy of the nodule on the right forearm showed an inflammatory pattern consistent with infection, but no organisms were identified. After treatment with parenteral antibiotics for 6 weeks, her systemic and ophthalmic findings resolved and her final left visual acuity was 20/40 (Fig 6).
Figure 6.
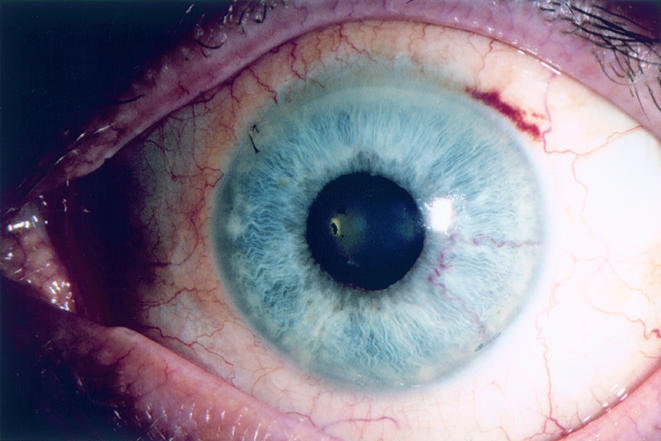
Case 3. Resolution of the iris nodule and anterior chamber reaction following a complete course of parenteral antibiotics. Final left visual acuity was 20/40.
CASES FROM THE LITERATURE
An additional 25 cases of infectious uveitis with iris nodules from the published literature were identified through a Medline search, and these reports reviewed.6–17,20–30 The reports varied widely in format and detail. In some cases key information was not available. However, some features were common to all or many of the reports. All of the cases reported unilateral eye disease and anterior chamber inflammation. Ten of 25 cases (40%) reported an initial diagnosis of inflammatory uveitis with immunosuppressive therapy that proved ineffective. Iris nodules were commonly reported to have features such as large size, a soft, whitish or creamy appearance, and/or many smaller white nodules scattered over the iris. However, most reports offered little qualitative description of the iris nodules. There was nearly equal prevalence of single and multiple nodules among the reviewed cases. A wide variety of bacterial and fungal species were reported to cause infectious uveitis with iris nodules. Mycobacterium tuberculosis, Mycobacterium leprae, Treponema pallidum, Coccidioides immitis, and C albicans collectively accounted for 64% of recently reported cases. The major features of each case are presented in Table 1.
Table 1.
| First author (year) | Organism | Systemic source | Method of diagnosis | Treatment | Outcome (final visual acuity if available) |
| Biswas J (1995) | M tuberculosis [2 patients] | Yes | Anterior chamber paracentesis | Systemic antibiotics, topical steroid | Enucleation [1 patient], recovery (20/80) [1 patient] |
| Rosen PH (1990) | M tuberculosis | No | Presumptive diagnosis | Systemic antibiotics | Recovery |
| Gain P (1994) | M tuberculosis | No | Iridectomy | Systemic antibiotics | Non-compliance, poor outcome |
| Walker C (1967) | “Tuberculosis” | No | Presumptive diagnosis | Systemic antibiotics | Recovery |
| Rosenbaum PS (1998) | M avium–intracellulare complex | Yes | Anterior chamber paracentesis, skin ulcer scrapings | Systemic antibiotics, topical steroid | Recovery then relapse with enucleation |
| Michelson JB (1979) | M leprae | Yes | Anterior chamber paracentesis | Systemic antibiotics, topical and subconjunctival steroids | Recovery |
| Messmer EM (1998) | M leprae | Yes | Anterior chamber paracentesis | Systemic antibiotics | Recovery |
| Daxecker (1997) | M leprae | Yes | Skin biopsy | Systemic antibiotics, topical steroid | Recovery |
| McCarron MJ (1998) | T pallidum | Yes | Anterior chamber paracentesis, skin biopsy, conjunctival biopsy | Systemic antibiotics | Recovery (20/25) |
| Schwartz LK (1980) | T pallidum | Yes | Blood test: FTA–abs | Systemic antibiotics, topical steroid | Unknown |
| Duffy RJ (1987) | R ricketsiae | Yes | Blood test: RMSF latex agglutination | Systemic antibiotics, topical steroid | Recovery (20/20) |
| Stokes DJ (1992) | P acnes | No | Iridectomy | Sub-conjuctival and topical antibiotics | Recovery (20/20) |
| Patel AS (1994) | Fusarium spp | Yes | Vitreous aspirate | Intravitreal, topical, and systemic antibiotics | Enucleation |
| Myers TD (2002) and Chalmers BE (1986) | A actinomycetemcomitans | Presumed | Anterior chamber paracentesis | Systemic antibiotics | Recovery (20/40) |
| Cunningham ET (1998) | C immitis* | Yes | Skin biopsy | Systemic antibiotics | Unknown |
| Cutler JE (1978) | C immitis* | Yes | Anterior chamber paracentesis | Intravitreal and intracameral antibiotics | Enucleation |
| Moorthy RS (1994) | C immitis* [3 patients] | No | Anterior chamber paracentesis, iridectomy | Systemic antibiotics | Enucleation [1 patient], undergoing therapy [2 patients] |
| Stone JL (1993) | C immitis* | Yes | Vitreous aspirate | Systemic and intraocular antibiotics | Enucleation |
| Stern JH (2001) | C albicans | Yes | Vitrectomy/vitreous aspirate, skin culture | Systemic antibiotics, vitrectomy, topical steroid | Recovery (hand movements) |
| Myers TD (2002) | C albicans | Yes | Anterior chamber paracentesis, vitreous aspirate | Systemic antibiotics, vitrectomy | Recovery (20/25) |
| Charles NC (1992) | C neoformans | Yes | Anterior chamber paracentesis | Vitrectomy, intravitreal antibiotics | Enucleation |
| Shyong MP (2000) | Penicillium spp | No | Anterior chamber paracentesis | Topical and intracameral antibiotics | Recovery |
| Myers TD (2002) | Corynebacterium spp | No | Anterior chamber paracentesis | Systemic antibiotics | Recovery |
| Gupta K (1986) | Possible HSV | No | Iris biopsy | Topical steroid | Recovery |
| Gass JDM (1973) | Possible HSV | No | Iris biopsy | Topical steroid | Recovery (20/70) |
*Additional reports in the literature published before dates covered by Medline search.
DISCUSSION
The differential diagnosis of iris nodules in the setting of uveitis is presented in Table 2.1–4,31–48 Infectious uveitis is an uncommon cause of iris nodules. Most cases of uveitis with iris nodules can be attributed to non-infectious entities, such as sarcoidosis, which are generally responsive to immunosuppressive therapy. While aetiologies of uveitis such as infection and malignancy are rare, they represent two important subsets of diseases that respond poorly to conventional immunosuppressive therapy. Indeed, many patients with iris nodules and infectious uveitis may have worsening of their disease if treated with immunosuppressive agents. On the other hand, early diagnosis and appropriate therapy may lead to more favourable outcomes. Thus, the importance of careful diagnosis cannot be overemphasised. Careful history taking, including a thorough review of systems to identify any systemic infectious illness, is a key part of making the correct diagnosis early in the patient’s disease course.
Table 2.
Differential diagnosis of iris nodules with uveitis
| Aetiology | Diagnostic clues |
| Sarcoidosis | Usually a bilateral chronic uveitis with mutton fat keratic precipitates. 11% of cases have Koeppe and/or Bussaca nodules.1,31 Excision of nodules may lead to clinical improvement.32 |
| Fuchs’ heterochromic iridocyclitis | Characteristic stellate keratic precipitates with or without marked heterochromia. 20–30% of cases have small, transparent iris stromal and pupillary border nodules. Nodules seen most often in darkly pigmented individuals.2,33 |
| Infectious uveitis | Usually associated with a history of systemic infectious disease or systemic symptoms such as fever and chills. May have large fluffy or creamy looking nodules. Responds poorly to corticosteroid treatment. |
| Multiple sclerosis | Granulomatous anterior and/or intermediate uveitis with iris nodules. History will often reveal neurological symptoms.4 |
| Vogt-Koyanagi-Harada syndrome | Prominent bilateral posterior segment inflammation often seen with poliosis, vitiligo, and alopecia. May have iris nodules and anterior chamber inflammation during the uveitic phase of the disease.3 |
| Metastatic neoplasm | Greyish white translucent nodules in the setting of malignancy elsewhere. May have other features such as hypopyon, hyphaema, secondary glaucoma, and iris atrophy.34 |
| Post-transplant lymphoproliferative disorder | Usually seen in immunosuppressed children and is often bilateral. Associated with Epstein-Barr virus exposure after organ transplantation.35–37 |
| Lymphoma and leukaemia | Seen in cases of systemic malignancy. May have hypopyon uveitis.38–40 |
| Foreign body | History of ocular trauma or ocular foreign body. May see foreign body in cornea or conjunctiva. Caterpillar hairs have been frequently reported as a cause of iris nodules.41 |
| Primary malignancy | Most common malignancies include melanoma, retinoblastoma, medulloepithelioma, and sarcoma. Pigmented or non-pigmented mass. Often found to have no response to steroids, refractory glaucoma, and hyphaema. Nodules may show growth over time.42–46 |
| Juvenile xanthogranuloma | Yellowish iris nodules often seen with a hyphaema and skin lesions.47 |
| Drug induced | Pinkish fleshy mass has been reported in association with propranolol.48 |
Table 1 outlines recently reported causes of infectious uveitis with iris nodules.6–17,20–30 The diagnosis in the vast majority of these cases relied on detailed medical history and anterior chamber paracentesis, after the discovery of an iris nodule with uveitis. Associations of uveitis, iris nodules, and organisms such as Coccidioides immitis and Mycobacterium leprae, are well described in the literature. Indeed, additional cases of C immitis associated iris nodules reported before the period included in the Medline database, may be found in the literature. Other associations are more tentative. The reported cases of herpes simplex virus associated uveitis with striking brownish iris nodules, after dendritic keratitis, are not definitive because no organism has been isolated by culture or clearly identified otherwise.29,30 In these cases, suggestive patient history combined with characteristic pathology was the basis of the association. Both cases were published before widespread use of the polymerase chain reaction (PCR) as a diagnostic method. Future reports might strengthen the proposed association through use of this technique.
Some diagnostic clues to an infectious aetiology for iris nodules with uveitis were common to the cases we reviewed.6–17,20–30 The most important clues were found on patient history. Many of the cases were felt to be due to metastatic rather than a primary infection. An apparent source of the micro-organism was usually uncovered in the history. Additionally, almost one half of the patients were referred to tertiary eye care facilities with a presumed non-infectious uveitis that had demonstrated a poor response to corticosteroid therapy. Many cases were therefore characterised as chronic and corticosteroid resistant. Every case was unilateral and characterised by a marked anterior chamber inflammatory response, often with associated vitreous inflammation. The iris nodules caused by infection were usually described as large, white, “fluffy” or “creamy” and/or lobulated, sometimes with many smaller background nodules. Findings of hyphaema or distortion of the anterior segment architecture were relatively uncharacteristic of infectious nodules and were more often described with neoplastic nodules.34,42 While elevated intraocular pressure was often noted with infectious uveitis, it was just as commonly seen with other aetiologies listed in Table 1 and was therefore not particularly helpful.
Anterior chamber paracentesis is an underutilised investigation. However, as illustrated by the cases treated at our institution, it is an invaluable tool in establishing a definitive diagnosis of infectious uveitis. This procedure can be conveniently performed in the outpatient setting, and has been shown to be safe in the hands of an experienced ophthalmologist.49 The information offered by an aqueous aspirate culture and stain, coupled with patient history and examination findings, can guide therapy and lead to appropriate treatment of disease beyond the eye.
Interestingly, one of the cases we present (case 2) had a recurrence of sterile uveitis 3 years after initially presenting with infectious uveitis. It is possible that her initial infection may have predisposed her to further episodes of non-infectious uveitis by: (1) leaving residual bacterial products such as peptidoglycan; (2) sensitising the immune system to antigens from an immune privileged site; or (3) favouring the deposition of circulating immune complexes as in the experimental Auer reaction.50
Nodules are an important diagnostic clue in the evaluation of uveitis. They should prompt a rededication to thorough review of systems and medical history. Specific symptoms relating to systemic infection, malignancy, and even drug side effects, as well as certain systemic inflammatory diseases, should be reviewed. Anterior chamber paracentesis can offer valuable information regarding the diagnosis and treatment of an infectious process. Because many patients with iris nodules and infectious uveitis do poorly with corticosteroid or other immunosuppressive therapy, early diagnosis and treatment may lead to more effective treatment and improve outcome.
Acknowledgments
Supported by in part by grants from Rosenfeld Family Fund and Research to Prevent Blindness.
REFERENCES
- 1.Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis.Am J Ophthalmol 1986;102:297–301. [DOI] [PubMed] [Google Scholar]
- 2.Rothova A, La Hey E, Baarsma GS, et al. Iris nodules in Fuchs’ heterochromic uveitis.Am J Ophthalmol 1994;118:338–42. [DOI] [PubMed] [Google Scholar]
- 3.Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome.Surv Ophthalmol 1995;39:265–92. [DOI] [PubMed] [Google Scholar]
- 4.Bachman DM, Rosenthal AR, Beckingsdale AB. Granulomatous uveitis in neurological disease.Br J Ophthalmol 1985;69:192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helm CJ, Holland GN. Ocular tuberculosis.Surv Ophthalmol 1993;38:229–56. [DOI] [PubMed] [Google Scholar]
- 6.Biswas J, Madhavan HN, Lingham G, et al. Intraocular tuberculosis, clinicopathologic study of five cases. Retina 1995;15:461–8. [PubMed] [Google Scholar]
- 7.Rosen PH, Spalton DJ, Graham EM. Intraocular tuberculosis.Eye 1990;4:486–92. [DOI] [PubMed] [Google Scholar]
- 8.McCarron MJ, Albert DM. Iridocyclitis and an iris mass associated with secondary syphilis.Ophthalmology 1998;91:1264–8. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz LK, O’Conner GR. Secondary syphilis with iris papules.Am J Ophthalmol 1980;90:380–4. [DOI] [PubMed] [Google Scholar]
- 10.Duffey RJ, Hammer ME. The ocular manifestations of rocky mountain spotted fever.Ann Ophthalmol 1987;19:301–6. [PubMed] [Google Scholar]
- 11.Charles NC, Boxrud CA, Small EA. Cryptococcosis of the anterior segment in acquired immune deficiency syndrome.Ophthalmology 1992;99:813–6. [DOI] [PubMed] [Google Scholar]
- 12.Moorthy RS, Rao NA, Sidikaro Y, et al. Coccidioidomycosis iridocyclitis.Ophthalmology 1994;101:1923–8. [DOI] [PubMed] [Google Scholar]
- 13.Stone JL, Kalina RE. Ocular coccidioidomycosis.Am J Ophthalmol 1993;116:249–50. [DOI] [PubMed] [Google Scholar]
- 14.Cutler JE, Binder PS, Paul O, et al. Metastatic coccidioidal endophthalmitis.Arch Ophthalmol 1978;96:689–91. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham ET, Seiff SR, Berger TG, et al. Intraocular coccidioidomycosis diagnosed by skin biopsy.Arch Ophthalmol 1998;116:674–7. [DOI] [PubMed] [Google Scholar]
- 16.Stern JH, Calvano C, Simon JW. Recurrent endogenous candidal endophthalmitis in a premature infant.J AAPOS 2001;5:50–1. [DOI] [PubMed] [Google Scholar]
- 17.Chalmers BE, Rosenbaum JT, Fraunfelder FT, et al. Iris manifestations of bacterial endocarditis.Arch Ophthalmol 1986;104:1548–9. [DOI] [PubMed] [Google Scholar]
- 18.Vandepitte J, De Geest H, Jousten P. Sub-acute bacterial endocarditis due to Actinobacillus actinomycetemcomitans.J Clin Pathol 1977;30:842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Affias S, West A, Stewart JW, et al. Actinobacillus actinomycetemcomitans endocarditis.Can Med Assoc J 1978;118:1256–60. [PMC free article] [PubMed] [Google Scholar]
- 20.Gain P, Mosnier JF, Gravelet C, et al. Tuberculose irienne. A propos d’une observation diagnostiquee par iridectomie.J Fr Ophtalmol 1994;17:525–8. [PubMed] [Google Scholar]
- 21.Walker C. Conglomerate tuberculosis of the iris with scleral perforation.Br J Ophthalmol 1967;51:256–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum PS, Mbekeani JN, Kress Y. Atypical mycobacterial panophthalmitis seen with iris nodules.Arch Ophthalmol 1998;116:1524–7. [DOI] [PubMed] [Google Scholar]
- 23.Michelson JB, Roth AM, Waring GO. Lepromatous iridocyclitis diagnosed by anterior chamber paracentesis.Am J Ophthalmol 1979;88:674–9. [DOI] [PubMed] [Google Scholar]
- 24.Messmer EM, Raisman MB, Foster CS. Lepromatous uveitis diagnosed by iris biopsy.Graefes Arch Clin Exp Ophthalmol 1998; 236:717–9. [DOI] [PubMed] [Google Scholar]
- 25.Daxecker F, Staudacher C. Ocular involvement in tuberculoid leprosy—a case report.Ophthalmologica 1997;211:305–7. [DOI] [PubMed] [Google Scholar]
- 26.Stokes DW, O’Day DM. Iris nodule and intralenticular abscess associated with propionibacterium acnes endophthalmitis.Arch Ophthalmol 1992;110:921–2. [DOI] [PubMed] [Google Scholar]
- 27.Patel AS, Hemady RK, Rodrigues M, et al. Endogenous fusarium endophthalmitis in a patient with acute lymphocytic leukemia.Am J Ophthalmol 1994;117:363–8. [DOI] [PubMed] [Google Scholar]
- 28.Shyong MP, Chen SJ, Lee FL, et al. Pseudophakic penicillium endophthalmitis.Chung Hua I Hsueh Tsa Chih (Taipei) 2000;63:770–73.11076435 [Google Scholar]
- 29.Gupta K, Hoepner JA, Streeten BW. Pseudomelanoma of the iris in herpes simplex keratoiritis.Ophthalmology 1986;93:1524–7. [DOI] [PubMed] [Google Scholar]
- 30.Gass JDM. Iris abscess simulating malignant melanoma.Arch Ophthalmol 1973;90:300–1. [DOI] [PubMed] [Google Scholar]
- 31.Obenauf CD, Shaw HE, Sydnor CF, et al. Sarcoidosis and its ophthalmic manifestations.Am J Ophthalmol 1978;86:648–55. [DOI] [PubMed] [Google Scholar]
- 32.Ocampo VV Jr, Foster CS, Baltatzis S. Surgical excision of iris nodules in the management of sarcoid uveitis.Ophthalmology 2001;108:1296–9. [DOI] [PubMed] [Google Scholar]
- 33.Goble RR, Murray PI. Fuchs’ heterochromic uveitis and sarcoidosis.Br J Ophthalmol 1995;79:1021–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman TR, Friedman AH. Metastatic carcinoma of the iris.Am J Ophthalmol 1975;80:947–52. [DOI] [PubMed] [Google Scholar]
- 35.Cho AS, Holland GN, Glasgow BJ, et al. Ocular involvement in patients with posttransplant lymphoproliferative disorder.Arch Ophthalmol 2001;119:183–9. [PubMed] [Google Scholar]
- 36.O’Hara M, Lloyd WC, Scribbick FW, et al. Latent intracellular Epstein-Barr virus DNA demonstrated in ocular posttransplant lymphoproliferative disorder mimicking granulomatous uveitis with iris nodules in a child.J AAPOS 2001;5:62–3. [DOI] [PubMed] [Google Scholar]
- 37.Cook T, Grostern RJ, Barney NP, et al. Posttransplant lymphoproliferative disorder initially presenting as iris mass and uveitis.Arch Ophthalmol 2001;119:768–70. [DOI] [PubMed] [Google Scholar]
- 38.Verity DH, Graham EM, Carr R, et al. Hypopyon uveitis and iris nodules in non-Hodgkin’s lymphoma: ocular relapse during systemic remission.Clin Oncol (R Coll Radiol) 2000;12:292–4. [DOI] [PubMed] [Google Scholar]
- 39.Jensen OA, Johansen S, Kiss K. Intraocular T-cell lymphoma mimicking a ring melanoma. First manifestation of a systemic disease. Report of a case and survey of the literature.Graefes Arch Exp Ophthalmol 1994;232:148–52. [DOI] [PubMed] [Google Scholar]
- 40.Ells A, Clarke WN, Noel LP. Pseudohypopyon in acute myelogenous leukemia.J Pediatr Ophthalmol Strabismus 1995;32:123–4. [DOI] [PubMed] [Google Scholar]
- 41.Cadera W, Pachtman MA, Fountain JA, et al. Ocular lesions caused by caterpillar hairs (ophthalmia nodosa).Can J Ophthalmol 1984;19:40–4. [PubMed] [Google Scholar]
- 42.Shields CL, Shields JA, Materin M, et al. Iris melanoma. Risk factors for metastasis in 169 consecutive patients.Ophthalmology 2000;108:172–8. [DOI] [PubMed] [Google Scholar]
- 43.Raju VK, Green WR. Reticulum cell sarcoma of the uvea.Ann Ophthalmol 1982;14:555–60. [PubMed] [Google Scholar]
- 44.Brownstein S, Barsoum-Homsy M, Conway VH, et al. Nonteratoid medulloepithelioma of the ciliary body.Ophthalmology 1984;91:1118–22. [DOI] [PubMed] [Google Scholar]
- 45.Girard B, Brezin A, Gaumond MC, et al. Retinoblastoma diffus—a propos d’une observation.Bull Soc Ophtalmol Fr 1989:89:25–30. [PubMed] [Google Scholar]
- 46.Bhatnagar R, Vine AK. Diffuse infiltrating retinoblastoma.Ophthalmology 1991;98:1657–61. [DOI] [PubMed] [Google Scholar]
- 47.Zamir E, Wang RC, Krishnakumar S, et al. Juvenile xanthogranuloma masquerading as pediatric chronic uveitis. A clinicopathologic study.Surv Ophthalmol 2001;46:164–71. [DOI] [PubMed] [Google Scholar]
- 48.Yeomans SM, Knox DL, Green WR, et al. Ocular inflammatory pseudotumor associated with propranolol therapy.Ophthalmology 1983;90:1422–5. [DOI] [PubMed] [Google Scholar]
- 49.Van der Lelij, Rothova A. Diagnostic anterior chamber paracentesis in uveitis: a safe procedure?Br J Ophthalmol 1997;81:976–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gamble CN, Aronson B, Brescia FS. Experimental uveitis II. The pathogenesis of recurrent immunologic (Auer) uveitis.Arch Ophthalmol 1970;84:331–41 [DOI] [PubMed] [Google Scholar]


